J Biomed 2016; 1:9-25. doi:10.7150/jbm.16945 This volume Cite
Research Paper
A New Endobronchial Ultrasound (EBUS) Application for Benign and Malignant Pericardial Effusion (PE) Aspiration: Transbronchial Pericardial Effusion Aspiration (TPEA) with a Regular EBUS Transbronchial (TBNA) Needle under Apneic Nasal Jet-Catheter Ventilation
1. Medical Clinic I, ''Fuerth'' Hospital, University of Erlangen, Fuerth, Germany.
2. Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
3. Department of General, Thoracic and Vascular Surgery, ''Fuerth Hospital'', University of Erlangen, Fuerth, Germany;
4. Department of Diagnostic and Interventional Radiology, Goethe University of Frankfurt, Frankfurt, Germany.
5. Professor and Vice-Chairman Co-Director: Thoracic Oncology Department of Medicine, University of Tennessee Graduate School of Medicine 1924 Alcoa Highway, U114 Knoxville, TN 37920, USA.
6. Director of Interventional Pulmonology, Walter Reed National Military Medical Center, Bethesda, MD, USA.
7. CardioThoracic Surgery Department, “Saint Luke” Private Hospital, Panorama, Thessaloniki, Greece.
8. Department of Respiratory Diseases, Changhai Hospital/First Affeliated Hospital of the Secondary Military Medical University, Shanghai, China.
Received 2016-8-20; Accepted 2016-9-15; Published 2016-9-20
Abstract
Background: Endobronchial Ultrasound (EBUS) is a state of the art diagnostic tool for the pulmonary physicians, especially for the diagnosis and staging of lung cancer. Pericardial effusion (PE) is examined regularly when either hemodynamic impairment occurs or the ventral PE seems to be at least of 2cm distance on echocardiography. Standard approach is the anterior pericardiocentesis. PE of less than 2cm is not approached in general. Objective: We sought to examine minimal invasively PE in an earlier stage with an aspiration through a regular EBUS-TBNA needle. Due to gravitation posterior PEs are earlier detectable than anterior PEs in a patient supine. Method: Although posterior PE is a contradiction for the standard approach used by cardiologists we punctured the PE from the distal left main or proximal lower lobe bronchus. For the first time we describe our algorithm of Transbronchial PE Aspiration (TPEA) by the usage of a regular EBUS-TBNA needle aspiration controlled by C-arm and regular transthoracic ultrasound. Results: We performed TPEA in deep sedation under nasal jet-ventilation in 10 patients without any severe complications even in anterior PE smaller than 20mm for early diagnosis and in some cases even for treatment in regards to hemodynamics. For the post-puncture period of follow-up over 60 days none of these patients had to be punctured again. Conclusion: Although TPEA is an uncommon and possibly more risky approach than the regular anterior pericardiocentesis it may give a chance to interventional pulmonologists to diagnose PE earlier than achieved by anterior transthoracic ultrasound. At the same time TPEA could offer a new window to the heart for several treatment options beside an effusion retrieval. We additionally build the bridge to possible other applications in different areas in future.
Keywords: pericardial effusion, C-arm, endobronchial ultrasound.
Introduction
Lung cancer is the leading cause of cancer death after prostate cancer in men and breast cancer in women.1 In the recent years there has been an evolution in the treatment of non-small cell lung cancer with targeted treatments being the tip of the arrow.2,3 In order to know if targeted treatment can be provided to patients, sufficient sample material is needed from the tumor upon diagnosis or re-biopsy.4 Currently cytologic or tissue sample can be used with different molecular analysis methods in order to identify possible tumor mutations.5-7 Pulmonary physicians have been using their own diagnostic tools for sample acquisition.8 In the past decade there has been an evolution in the usage of radial and linear endobronchial ultrasound (EBUS).9 The EBUS diagnostic tool is used primarily for lung cancer staging and lung cancer diagnoses in cases where bronchoscopy cannot assist. The ultrasound equipment can observe several structures of the mediastinum; lymph nodes, tumors, vessels, pericardium and the heart. Thrombi can be observed within the vessels and also PE. It is known that the EBUS needles used worldwide are 22G and 21G.10 These needles have a stylet inside which is removed before puncturing a targeted structure. In the recent years the method of sample acquisition has been reevaluated and changed in order to acquire larger samples.9 Moreover; different techniques have been used from the cytopathologists in order to enhance the diagnostic procedures of the EBUS sample indifferent of the needle diameter.11-14 In regards to a therapeutical approach the EBUS system has been used for the application of cytostatic drugs within lymph nodes and tumor, as a method of local treatment for lung cancer.15,16 The EBUS system is also able to identify thrombus within vessels and pulmonary hypertension.17,18 (Figs. 1-2)
B. Maisch engaged in treatment of PE over several decades and one of the very few experienced users of another yet seldomly used endoscopic approach - the pericardioscopy33 - complained that in the new (second) European Society Cardiology (ESC) guideline in 2015 on pericardial diseases34 little has changed since the first guideline in 2004 with respect to the etiological classification of any form of pericarditis or PE: 'By classifying pericardial syndromes usually as idiopathic while a malignant or bacterial cause has been excluded, the real cause is in some cases overlooked.' His own registry published in 2013 showed a rate of idiopathic PEs of less than 10% when all techniques including pericardioscopy were used.33 This is clearly much better than the rate accepted in the literature for recurrent idiopathic pericarditis with 30-50%.35
As the first author is working as interventional pulmonologist and interventional cardiologist and furthermore was trained by B. Maisch at the University of Marburg, Germany as student we sought to approach earlier developing PE with less than 2cm in regards to identification of etiologies. However, pericardioscopy used in the days of B. Maisch is clearly not available in most cases which is not true for endobronchial ultrasound (EBUS): More than hundred systems are available on the German market whereas we only have the knowledge of four sites in Germany offering / publishing about pericardioscopy in the last decade. One has to ask why there is no rapid development of new techniques: Most of the 'under'-diagnosed idiopathic PEs appeared as if there were no impact on morbidity and mortality. The regular today's non-invasive diagnosis PE in the light of pericarditis / perimyocarditis shows an excellent prognosis following the actual guideline without the need to go for invasive diagnostics.36
Furthermore, the actual ESC guideline even reduces the need for rapid / urgent therapeutical (and diagnostical) approach to PEs (PEs) less than 10mm by adding a value of -1 to a triage tamponade score whereas PEs > 20mm results in an addition of 3 points and malignancy or proven tuberculosis shows an addition of 2 points. By reaching a value of 6 there is definite and immediate need for pericardiocentesis. Nowadays, the data are limited regarding the impact of the etiology of so called idiopathic pericarditis (or in general non-bacterial non-malignant PEs targeted by the DROP study37) which is further more depending on external factors like site of diagnosis (with e.g. different experience in invasive diagnostics), origin (with e.g. different infectious disease prevalence), age and sex (e.g. in regards to prevalence of collagenosis or forms of pulmonary hypertension) and comorbidities (with e.g. different prevalence of malignancies). The actual guideline34 follows an algorithm in which beside hemodynamic relevant PEs in general only effusions with a separation in diastolic phase of at least 20 mm are valuable targets if one can approach these PEs anteriorly. Although this wise algorithm has been clinically developed over the last 2 decades it is of note that there are several situations with possible initial PEs less than 20mm and of no obvious clinical hemodynamic relevance which can turn into dangerous situations in some instances quite rapidly. There is no regular solution for isolated posterior PE (behind the left atrium and ventricle). Furthermore, due to gravitation a freely moving PE is expected to be of larger diameter than an anterior PE especially in the early phases of a progressing PE.
As it has been previously proposed that the EBUS system could be used for PE aspiration the authors present their methodology in the current research.19
Patients and Methods
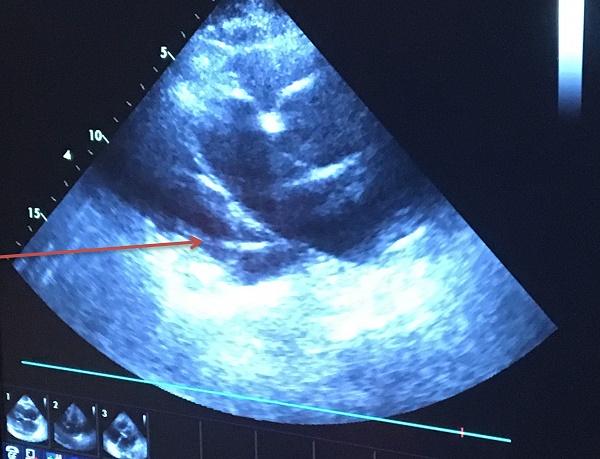
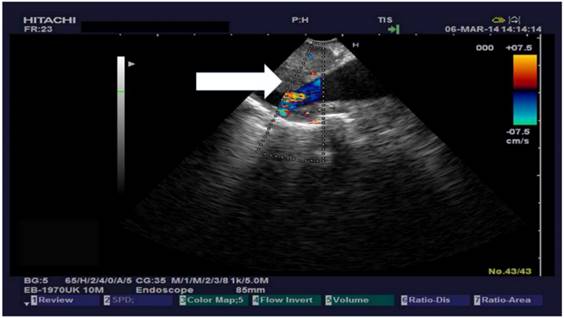
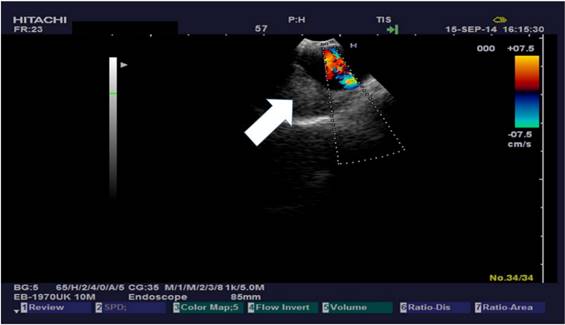
The research study was approved by our investigational review board (IRB) “G. Papanikolaou” General Hospital. We used a Fujinon, Olympus and Pentax linear endobronchial ultrasound system at different sites. The operators were Wolfgang Hohenforst-Schmidt (Fujinon system in Coburg Hospital, Olympus system in Fuerth Hospital) (Fig. 3.) and Paul Zarogoulidis (Pentax system in Thessaloniki) (Fig. 4.). Additionally, a cardiac ultrasound (U/S) system (Fig. 5-6.) and a C-Arm system were used (Fig. 7-8.). We used the cardiac U/S as an initial evaluation of the PE. Furthermore, its use offers the opportunity to evaluate real time changes of the cardiac hemodynamics due to reduction of PE while aspirating. During the procedure of aspiration, we used additionally a C-Arm system to present the location of the EBUS system within the thorax. The aspiration of PE has been previously proposed by the authors and with this research we applied the technique according to the approved protocol in a small number of patients with benign and malignant PE (Fig. 9-12.). 19 Ten patients were included in our study, three with benign and seven with malignant PEs. (TABLE 1.) The PE in all cases was sent for cytological evaluation (Fig. 13-14). The diagnosis was made upon this evaluation including the clinical setting.
Thrombus within pulmonary artery.
Thrombus within pulmonary artery.
Olympus ebus with needle.
Pentax ebus with needle.

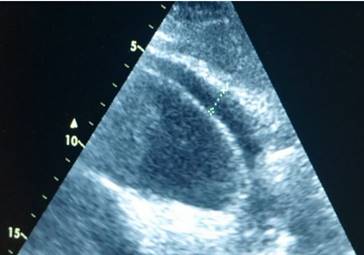
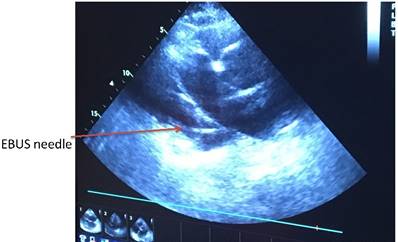
Echo control from subxiphoidal position during TPEA.
Echo control from subxiphoidal position during TPEA.
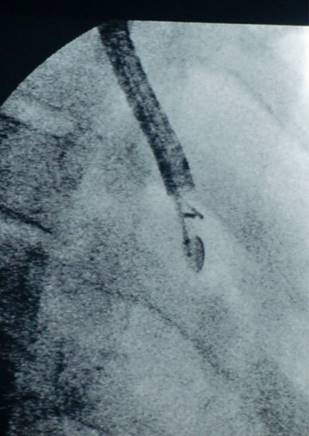
C-arm.
C-arm and ebus head within the chest cavity.
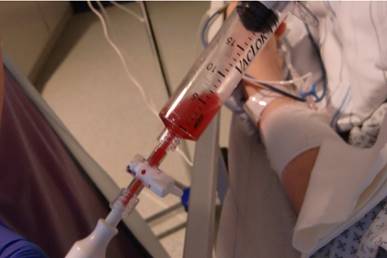
Fluid aspirate.
Patients' characteristics
| PE before TPEA | PE after TPEA | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sex | age at ex. | BMI | known medical condition | volume retrieved | aspiration time (min) | PE anterior (mm) | PE posterior seen in EBUS (mm) | PE anterior (mm) | PE posterior seen in EBUS (mm) | cytology | final diagnosis of PE | increase of 6 min walking distance (m) | FU max. PE in mm | died after FU 60days | Pericardiocentesis after FU | Remarks |
| f | 55 | 25 | Sjögren's Syndrome | 600 | 30 | 12 | 17 | 6 | 9 | benign | reference centre: No lymphoma | 70 | anterior PE 10 | no | no | discrete arterial bleeding of pericardial sac |
| f | 69 | 35,1 | Morbus Fabry | 150 | 18 | 10 | 16 | 8 | 14 | benign | benign | 18 | anterior PE 14 | no | no | hemodynamic instable during TPEA |
| m | 72 | 23,2 | Mesothelioma Stage IV | 470 | 58 | 25 | 30 | 14 | 15 | malignant | mesothelioma | 71 | anterior PE 15 | no | no | intrapericardial pressure measurement via EBUS needle |
| m | 76 | 31 | NSCLC Stage IV | 350 | 40 | 18 | 15 | 14 | 15 | benign | benign | 0 | anterior PE 14 | no | no | septated PE |
| f | 59 | 22 | Sjögren's Syndrome | 450 | 58 | 14 | 22 | 8 | 14 | benign | benign | 65 | anterior PE 14 | no | no | intrapericardial pressure measurement via EBUS needle |
| f | 52 | 20,2 | SCLC Stage IV | 180 | 20 | 14 | 19 | 12 | 11 | malignant | SCLC | 49 | anterior PE 20 | 183 days after TPEA | yes | none |
| m | 70 | 16,2 | NSCLC Stage IV | 20 | 15 | 22 | 25 | 22 | 25 | bloody , hb 5g/dl | NSCLC | 0 | anterior PE 22 | 89 days after TPEA | no | septated PE, bloody |
| m | 69 | 23 | NSCLC Stage IV | 180 | 32 | 23 | 28 | 18 | 24 | malignant | malignant | 33 | anterior PE 24 | 76 days after TPEA | yes | none |
| m | 79 | 23,3 | NSCLC Stage IV | 210 | 26 | 22 | 25 | 16 | 20 | malignant | malignant | 39 | anterior PE 18 | 101 days after TPEA | no | none |
| f | 79 | 21 | NSCLC Stage IV | 190 | 36 | 18 | 25 | 16 | 24 | benign | benign | 25 | anterior PE 25 | 69 days after TPEA | yes | none |
Fluid aspirate.
Fluid aspirate.
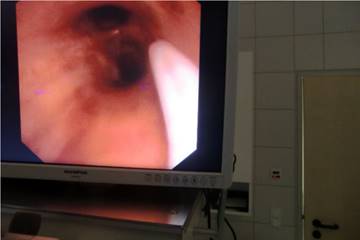
Endoscopic figure during puncture.
Pathology figure.
Pathology figure.
Jet-catheter.
The approved and examined protocol consisted of the following steps and aspects, the hereby reported measurements are included in this paragraph:
1. The day before (d-1 examination) TPEA was performed and all patients underwent a regular diagnostic flexible bronchoscopy during which we individually tested the feasibility in regards to anatomical and hemodynamical considerations of an application of nasal jet-catheter ventilation under deep sedation with propofol and remifentanil in patients with an obvious PE. (Fig. 15.) Propofol was applied by a perfusor given at a predefined concentration and flow throughout the whole procedure.20 Manual small boli of propofol were not allowed during the first d-1 examination. The initiation of the sedation was always done by midazolam up to 5mg iv. With this titration concept we aimed to find out the lowest sedation dosing in order to minimize the negative hemodynamic effects (as mentioned below). Of note is the fact that all patients received both drugs in the above-mentioned manner to guarantee the absolute control of the lung movement: Complete intermediate apnea especially for puncturing was essential. To guarantee a minimal blood pressure of min. systolic >100mmHg resp. min. diastolic > 60mmHg we applied a sympathomimetic drug called Akrinor (a mixture of cafedrine and theodrenalin, only allowed in Germany; in Greece we used noradrenalin as published before 20 ) and regular saline infusion via 2 iv lines as volume expansion increases the cardiac output (CO) in PE situations.21 Oxygen saturation was measured by a fingertip, oxygen delivery was controlled by jet-catheter ventilation setting. During the d-1 examination we cleaned the bronchial airways in order to analyse possible bacterial specimen as a possible source of bacterial transfection through the bronchial wall into the pericardial space by puncturing. A massive prurulent situation in the bronchial airways was an exclusion criterion for this approach.
2. After this cleaning procedure we started to apply i.v ceftriaxone 2g once daily for overall three days until the day after the TPEA (d+1). If necessary, we would have applied an antibiotic suitable to treat the analysed specimen in a calculated manner. Of note is the fact that there was no sign of even a slight infection due to this transbronchial needle puncture into the pericardial sac in all ten cases. Therefore, we only applied ceftriaxone 2g i.v. over three days in all ten cases.
3. During TPEA the same way of sedation and nasal jet-catheter ventilation as on the d-1 examination were applied. Remifentanil was only used during TPEA when at the d-1 examination an unwanted lung movement despite the application of propofol in the above-mentioned manner was observed. Manual small boli of propofol (10 mg) were only allowed during the TPEA. A regular non-invasive hemodynamic control was applied with ECG, automatic blood pressure measurements and oxygen saturation measurements at a finger's tip. An oxygen-saturation below 88% before the examination was not allowed and would have caused immediate interruption of the approach. This has happened only once during the procedure in a heavily overweighted patient. Detailed observation of the heart movement and several measurements especially in regards to PE reduction were performed by transthoracic echocardiography (echo) additionally. The reference parameter of first choice (as explained below) was the distance between the free anterior wall of the right ventricle in end-diastole (RVAWed) and pericardial sac in the subxiphoid view. Therefore, an experienced echocardiographer was always the second examiner. Furthermore, at least one examiner had to be an interventional cardiologist with experience in regular pericardial drainage. All medical equipment to treat accurately an acute pericardial tamponade, a resuscitation including intubation or an intrabronchial bleeding event had to be in the room readily available. The examination was always accompanied by 2 bronchoscopy nurses. Thoracic fluoroscopy was applied from time to time to control the needle position and to exclude any possible new infiltration (due to theoretical pulmonary parenchymal bleeding) or (mediastinal) pneumothorax. Systemic blood gas analyses and at least once initial analyses of the hemoglobin content of the aspirate (if reddish) were measured with a POCT analyser aside. Capillary sytemic paO2 values below 50mmHg and paCO2 values over 80mmHg and elevation of Hb content in the aspirate (or changing of the aspirate's colour) would have caused immediate interruption. After retracting the needle out of the pericardial sac midazolam-antagonist (anexate) was applied. The hemodynamically stable and awake patient was observed for 2 hours near the intervention ward where another echo and regular throracical x-ray in standing position (XR) were performed before transferal to his normal ward. Of note is the fact that except for one case in a heavily overweighted patient to whom we had to stop early the aspiration procedure, all the patients were hemodynamically ameliorated with reduced dyspnea and increased 6-minutes walking distance (0 to 71 meters, mean + 37 meters) comparing the distances on d-1 to d+1. The aspirate volume was between 20 and 600 ml, mean 280 ml. The time needed for a certain volume amount depended on the needle position and the amount of pericardial fluid (Fig. 16). With an unblocked free needle and a widely enlarged pericardial sac due to overdistension our observation was that the aspiration was faster in comparison to smaller PEs. We have not observed any influence to septation in regards to velocity of needle aspiration. However; it has to be mentioned that there was only one patient with a slightly septated PE. We stopped all procedures in less than an hour after initiating the penetration of the needle into the pericardial sac. The aspiration itself was done by a regular suction syringe prepared with maximal underpressure before connecting the needle's proximal end. We cleared this needle in a repeated manner and performed cytopathological evaluation afterwards.
4. The day after the TPEA another echo and XR were performed. Daily blood specimen for examination of infection signs (CRP, leucocyte count) were taken on d-1 until d+1 and the day before discharge. Of note, there was no delayed discharge due to the procedure. There was no lab-sign of bacterial infection in all ten cases.
5. Included were only patients with a chronical PE of either diagnostic interest or treatment necessities due to chronical hemodynamic impairment. In contrast to this aspect any signs of acute hemodynamical instability typically seen in acute tamponade were an exclusion criterion as well as impossible rapid jet-catheter application due to anatomical issues. Patient with allergies against the drugs or materials applied in our interventional bronchoscopy suite were excluded. Any drug affecting thrombocyte function or coagulation except for aspirine up to 100mg daily lead to an exclusion from the protocol. As this study was intended to be an all-comers study there was no exclusion criteria regarding the age (55-72 years, mean 68 years), body mass index (16,2 - 35,1 kg/m2, mean 24 kg/m2) or absolute weight (59-122kg, mean 79kg), cardiac or pulmonary diseases except for prediagnosed infectious diseases not only in the lung. Hemodynamic instability (e.g. inability to lie down on the bronchoscopy table in a calm manner without anxiety) or oxygen dependency over 3l/min in an upright position before examination was an exclusion criterion.
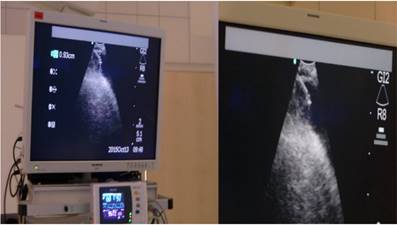
Heart ultrasound by EBUS and pericardial effusion with a TBNA-needle in it.
Results
Except for one patient with the highest BMI (35,1 kg/m2) and instable hemodynamic profile during TPEA all of the procedures have been accomplished without any complication mentioned above in the intended way - e.g. diagnostic approach of a PE smaller than 2cm anteriorly and/or hemodynamic relief. In another patient we stopped the procedure after retrieving 600ml of PE as the colour and the content of the analysed PE fluid changed to a significant higher hemoglobin level. We followed all 10 patients for 60 days by telephone calls and echo examinations on day 30 and day 60. Of the 10 patients 7 patients were in a palliative status. Until now 5 patients are still alive, 3 with benign disease due to 2 Sjögren's syndromes and one Morbus Fabry, one with mesothelioma and one with NSCLC causing PE. 5 patients with malignant PE died between 89 and 183 days after the TPEA procedure. Their deaths were related to expected biology but not linked to any aspect of the performed TPEA. There was no procedure related adverse event except for one hemodynamic instable patient during TPEA. Of note is the fact that all of the 10 patients had a certain relapse of PE but in general to a lesser extent during the 60 days of follow-up. Therefore, none of the patients during the follow-up period had to be punctured again. However, 3 patients with malignant disease had to be punctured again (this time by pericardiocentesis) due to hemodynamic considerations after the follow-up period. All of these 3 patients belonged to the group of 5 patients with malignant disease dying between 89 to 183 days after the TPEA procedure.
Discussion
The linear endobronchial ultrasound diagnostic tool has been proven through the years to be an efficient and safe tool. In several cases general anesthesia has been used, while in other cases only sedation in order to perform the examination was enough. The status of sedation is decided by the operator on a case by case scenario. This depends on the examination goal itself, technical and economical aspects, experience and institutional habits as well.
In contrast to the above-mentioned aspects for the patients referred to examination due to PE in this study we used exceptionally sedation protocols without relaxation but with complete apneic ventilation for optimal control of the lung and mediastinal movement with a nasal jet-catheter as recently reported.20 (Fig. 12). The reason for this decision was made/ based on upon 3 considerations (I-III):
I)A)
Patients with PE have a limited adaptability to hemodynamic changes, especially in regards to systemic blood pressure and heart frequency. Using the sedation and relaxation, the impact of blood pressure during the initiation of an examination is higher than using just the sedation. In regards to pathophysiology PE represents a status in which the organism tries to adapt to the impaired cardiac output due to reduced filling volumes by elevation of heart frequency. PE itself leads to a reduced inflow especially early in the right chambers during the initial process of an enlarging PE. The hemodynamic function of the right ventricle (RV) with its typical volume function in the cardiopulmonary circuit is very early negatively affected during the development of this disease. This is different to the, in general four times more powerful, left ventricle (LV) situated posteriorly in the thorax in sagital axis. During increased heart rates, the diastole as the filling time of the ventricles, is profoundly reduced, which impairs even more the already reduced filling adaptability of the RV due to anterior PE. The reduced diastolic filling of the LV leads to the impairment of systemic perfusion by reduced stroke volume (SV). The organism reacts by elevation of the heart rate (HR) as cardiac output (CO) is defined by CO = SV*HR which is the same for LV and RV. The first organ to be affected by reduced CO is again the heart itself due to coronary perfusion applied in diastole as the ostia of the coronary arteries origins directly above the level of the aortic valve. As SV is either a function of (predominantly) power (for the LV) or of (predominantly) volume (for the RV) the maintenance of either function depends on a certain coronary perfusion of the both chambers. Therefore, in acute tamponade the deadly risk is finally a break-down in minimal coronary perfusion. This is different to chronical PE as mentioned below. Of interesting note is the rarely described fact that contents of the pericardial fluid regulate the coronary arterial tone.22 Furthermore increasing PE has to be understood as a continuous affection of the filling pressure of all chambers which in the end leads always to a certain hemodynamic impairment.23 This hemodynamic impairment can be masked even in very large PEs in the case of so called (chronical) low-pressure tamponade.24 Moreover; in acute life-threatening tamponade, mostly with PE diameters > 2cm, however these (life-threatening acute) PEs could be smaller than in the above mentioned situation. Although different signs in echography are very valuable (like PE diameter or chamber collapsibility) from a clinical point of view the major cornerstone is clinically relevant hemodynamic impairment. The amount of hemodynamically acceptable large PE is a function of intrapericardial pressure influenced by the volume of PE and the actual compliance of the pericardial sac.25 Both values are opposed by the filling pressures especially of the atria which are defined by myocardial structural patterns, vitia, heart rhythm and frequency, and finally ejection fraction in addition to fluid properties of human blood. Due to interventricular dependency on a case-by-case scenario it is impossible to presume the exact individual threshold of a near-death-defining heart rate or blood pressure. Therefore, we did not define such values for the protocol as exclusion criteria.
I)B)
Another fact is the point that the RV anterior space is limited due to the retrosternal space which is in general smaller than the space behind the left ventricle. Due to the anatomy of the pericardial sac the limitations of overdistensibility is also a function of time, as the pericardial sac can adapt successfully to a high volume of pericardial fluid over a longer time - the main difference between chronical PE and acute pericardial tamponade is therefore the situative adaptability of pericardial compliance.26 The pericardial sac has a visceral and parietal leafs which guarantee a high adaptability of distension over time. This means in the end that even a slowly emerging PE with an overdistensed pericardial sac will affect rather the RV more than the LV due to different space limitation of both ventricles. The same mechanistic problem of a primarily reduced RV filling will lead to a different clinical appearance: In chronical PE the final deadly risk could be a critical reduced systemic perfusion of non-cardiac organs as kidney, liver or lung due to chronical right heart insufficiency. However; in this setting of an EBUS approach towards a PE the mechanics of a chronical PE could be converted into the symptoms of an acute pericardial tamponade with a break-down of coronary perfusion by applying too aggressive relaxation including sedation protocols as thoroughly explained above and below. Of note is the fact that sometimes different areas of pericardial sac fluid do not interact with each other due to intrapericardial adhesions. Therefore, successful reduction of PE posterior or lateral to the LV does not guarantee in all cases a reduction of the same fluid affecting the RV. Another point is the fact that due to anatomy the spatial effect of the reduction of a PE is obviously depending on the patients' position. During EBUS-TPEA the posterolateral area of the LV is positioned more posterior than the anterior space of the RV. Therefore, free pericardial fluids follow the gravitation forces towards the posterior space around the heart. This space will not be reduced in diameter over a long time although aspiration occurs exactly at this site. This is another reason why the intrapericardial distance should be measured anteriorly to the RV.
Taken all these considerations into account it was clear for us that we had to control during the examination the success of a TPEA by documentation of the pericardial space anteriorly to the RV. Therefore, a second examiner experienced as echographer was essentially needed to perform these procedures. Of course, complete non-invasive hemodynamic control was guaranteed the whole time and implemented into the algorithm.
II)
A second consideration was precluded towards the EBUS-device and the application itself: In general, the easiest way to ventilate a person while introducing an EBUS intrabronchially is a function of the anatomical shape of each EBUS, especially the volume of the inserted 'foreign' body: The bigger the 'foreign body' EBUS, the more ventilation efforts have to be applied which affect negatively the already severely impaired hemodynamic situation. Although we do not have exact measurements of the above-mentioned systems, the Fujinon system is believed to have a small advantage in regards to this consideration as it is well known and accepted that the Fujinon head is smaller than the heads of the 2 other systems. 27
Four punctures of PE were performed during the engagement as consultant interventional pulmonology and cardiology of the first author in Coburg Hospital with a Fujinon system, three additional punctures were performed in Fuerth Hospital with an Olypus system and another 3 were performed by the second author with a Pentax system in Thessaloniki. Personal experience of the first author gives hints towards this small advantage of the Fujinon system in regards to this study. Furthermore, different needles for EBUS-TBNA are or will be available on different markets soon. Of note is the fact that this study was performed only with the Olympus needle which has a certain difference in comparison to the other needles (sold by Cook company for example): The Olympus needle consists merely of a plastic catheter with a plastic tube ending at the tip of this needle which is metallic. The new needles of Cook and other companies consist of more metallic properties especially in the needle mechanism itself. The advantage of these needles is believed to ease the introduction of the (more rigid) needle in a hardened malignant lymph node - this is sometimes called the 'punsh' or 'jabbing' technique in regular EBUS-TBNA for malignant lymph nodes. In our approach the somehow 'soft(er)' and 'more flexible' Olympus needle showed an advantage in our opinion. If we can control the movement of lung and mediastinum very strictly by applying an apneic jet-catheter ventilation, as published before 20, in contrary, the movement of the heart is almost uncontrollable. Therefore, we took into account that a contact between needle tip and visceral leaf of the pericardial sac could occur. This was a clear safety issue as the pericardial leaf of this sac is defining the outer border of the myocardial parenchyma and reflects the zone of macrovessel location. Puncturing or even close 'scratching' contact of such a macrovessel (which can be detected as well by EBUS) could lead to arterial bleeding into the PE. This arterial bleeding of a macrovessel with signs of rapid increasing PE would have severe death-threatening consequences due to paralleled reduced myocardial perfusion with the event of reduced ischemic ventricular function distal to the leakage of the macrovessel.
III)
A third consideration was regarding the special anatomical situation: The distal left main bronchus is very close to pericardial space with a distance less than 10mm when passing through the bronchial wall. The visibility of the neighboured cardiac structures is extremely good and much better than any visualistion during a standard transthoracic cardiac approach - this is the main advantage of this hereby first described method. As the head of the EBUS is positioned along the bronchus downwards to the left feet and the angulation of the needle insertion is 30-40 degrees different to this direction towards the mediastinum in total one has a direction of the needle nearly tangential to the posterolateral part of the left ventricle. This results in an intrapericardial nearly tangential positioning of the EBUS-TBNA needle in regards to the axis of lateroposterior wall of the LV at the level of distal left main bronchus. Again, this is a safety issue as this direction does (nearly) not oppose LV myocardium as long as one has a certain amount of pericardial space which is depending on the amount of local effusion. Of note is the fact that it has to be in the DISTAL left main bronchus or even proximal left lower lobe (entrance): The level at this insertion point for transbronchial approach of the pericardial sac is farer away of the ostia of descending mainstem of circumflex coronary artery (RCX) in comparison to the level for example near to the main carina. The diameters of the epicardial macrovessels will be even more reduced the further they are away from their origins. In reality there may be only interference with branches of the RCX.
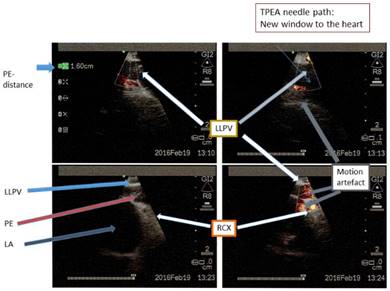
Taken all these 3 considerations into account the developed protocol allowed us to perform in a - so far - unproblematic manner the Transbronchial PE Aspiration (TPEA) using a regular EBUS-TBNA needle inserted at the level of distal left main bronchus or below: The new window to the heart is framed and limited by the ostia of the left pulmonary veins (Fig. 17-18), the EBUS-head is positioned very near to the level of left lower pulmonary vein and has to be rotated anterior to medial to avoid puncturing of this structure. The use of nasal jet-catheter ventilation with sedation only offers the best balance between controlled apneic ventilation for interventional pulmonology with the smallest affection of already limited cardiac output and essential control of any (unwanted) lung or mediastinal movement. We have never expected to use rigid bronchoscopy with essential relaxation nor conventional ventilation as it is well described that jet-ventilation (here applied as nasal jet-ventilation) even in worse situations like acute lung injury offers superior efficacy in regards to oxygenation at reduced carbon-dioxide levels in the pulmonary arterial bed (paCO2) allowing to reduce mean airway pressure with reduced to absolutely no impairment of hemodynamics in comparison to conventional ventilation.28-32 Although we have not compared different EBUS TBNA-needles the preferred Olympus needle had in this series of 10 patients an acceptable flexibility in regards to unavoidable contacts to the epicardial leaf of the pericardial sac with the risk of puncturing myocardial structures and inducing systemic arterial bleeding into the pericardial sac. This method of aspiration was observed to be safe in nearly all cases, even for intermediate PEs. The safety is based on the technical aspects of the applied algorithm. We propose that this method could be used by the pulmonary physicians, with the support of an experienced echographer during the procedure. This method could have the advantage of less invasiveness in comparison to the transcutaneous chest wall catheters being used by the cardiologist or radiologist: We were not in danger of passing by structures like liver, colon or gut. Up to now posterior effusion is regarded a contraindication as transcutaneous drainage target, however; for us as interventional pulmonologist it is essential. The main risk is bacterial infection of the pericardial sac for the TPEA approach. However; it can be treated effectively by antibiotics. We have to remember that after millions of mediastinal lymph node punctures worldwide via the same instrument and anatomical structure mediastinal infections are not an issue. The main advantage in comparison to other techniques is the small distance towards the pericardial sac and the excellent visibility of the puncture route. We have achieved already 600ml aspiration volume in 30 minutes without any problems in 6 of the above-mentioned cases following the explained algorithm. In regards to accepted periprocedural morbidity and mortality the risk of a pericardiocentesis is less than 1%. However; this pericardiocentesis will be applied especially in PEs > 20mm. Although we have performed only 10 cases so far (and 6 of them in moderate PEs with less than 2cm PE distance anterior) without any problems following this algorithm, the rate of major events is not yet defined. Our feeling is that this technique is suitable to apply for an experienced interventional pulmonologist with comparable risks to the above-mentioned method.
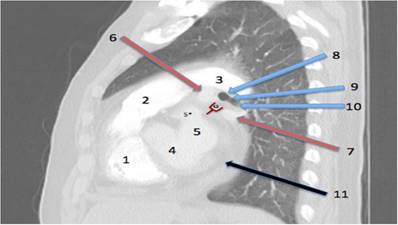
Anatomy of the procedure. The New Window to the Heart: Point of entry between (or often below) the pulmonary veins. Lateral view looking from the right side into the left hilum. 1= right ventricle. 2= pulmonary artery main stem. 3= left pulmonary artery. 4= left ventricle. 5= left atrium. 5*=left atrium appendage. 6= left upper pulmonary vein. 7= left lower pulmonary vein. 8=distal left main bronchus. 9=lower lobe carina to proximal left lower lobe. 10=lower lobe entrance. 11=pericardial effusion. 12=a new window to the heart situated near to (or below) the left lower pulmonary vein.
Needle in the pericardial sac. This is second examination for TPEA.
Conclusion and outlook
There are some clinical situations appearing with the main symptom PE in which TPEA could be implemented with a certain specific value. Due to the main advantage of the TPEA procedure - approaching early posterior PE less than 2cm - in comparison to the standard approach of (anterior) pericardiocentesis these special situations (I-II) are for our understanding the following:
I) Diagnostic considerations
a) Uremic pericarditis 38
Although this is nowadays seldomly perceived the addition of reduced hemodynamics in a patient with the need of dialysis permanently under danger of volume overfilling could be a situation where early minimal invasive drainage by TPEA could be an advantage. Firstly, in uremic pericarditis PE could be difficult to perceive clinically: Despite heart failure symptoms, there is no elevation of atrial natriuretic peptide and although coronary blood flow is reduced, there is no sign of myocardial ischemia. This could mean that for instance a PE diagnosed by EBUS a TPEA in these patients could be beneficial as uremic pericarditis could convert quite rapidly to tamponade. Secondly, intensive dialysis, (e.g. hemodialysis), is necessary for 5-7 days, performed without anticoagulation to prevent pericardial bleeding and tamponade, and with little or no fluid removal to avoid hypotension and cardiovascular collapse. In contrary to this approach with TPEA we could be much faster needing only one hour (or even less) to reduce the amount of PE by aspiration up to 600 ml (or even more). Thirdly, in a study by De Pace et al. beyond other blood and hemodynamic parameters large PE on echocardiography were predictors of poor response to intensive dialysis therapy.39 As we can reduce the volume of PE quite fast the specific treatment with dialysis could be eased, fastened or even avoided. Fourthly, although colchicine nowadays is one of the best options to treat PEs medically Busemeier et al.40 treated 45 patients with intractable uremic pericarditis unresponsive to intensive dialysis therapy with intrapericardial instillation of 100 mg of triamcinolone hexacetonide. All patients responded to treatment immediately and only 1 patient had recurrence of pericarditis. This could be easily achieved with TPEA as well.
b) Purulent pericarditis
Although this etiology clearly depends on origin, environmental and case-based factors it is still one of the most dangerous pericardial diseases with a high mortality rate if diagnosed too late or even underdiagnosed.41-45
Of note is the fact that beside (hopefully targeted) systemic antimicrobiotical therapy, the aspiration and evacuation of such pericardial fluid is urgently needed to reduce the risk of constrictive pericarditis which can follow very fast after purulent pericarditis.46 This etiology could serve as a major morbidity with really unexpected organism in immunocompromised or -competent patients.47,48 In immunocompetent situations for systemic empiric treatment one should be guided by local resistance patterns.49 It is reported that in comorbid patients (e.g. diabetes mellitus, prexisting PE, rheumatoid arthritis, obesity, etc.) with community acquired pneumonia complicated with purulent pericarditis one of the most frequent causative agents is streptococcus pneumonia. Any delay of definite diagnosis will increase the even high mortality rate up to 40% due to cardiac tamponade beside septic shock. The very early diagnosis and therapy by pericardiocentesis (or an alternative aspiration method) including antibiotics are essential to reduce the high mortality rate.50 This could be achieved as well with TPEA. Current data indicate that when there is a cyst in the mediastinum, diagnostic aspiration with the EBUS needle could cause microbial inoculation from the airways, however; there are cases where the EBUS method for diagnosis of cystic structures is very helpful. Such case could be a patient that has contraindications or comorbities for diagnostic thoracic surgery. Also, an assessment and aspiration with the ebus needle could be of extreme importance in order to relieve that patient of a large burbeon of pus that is concentrated within a thoracic abcess before surgery is necessary, or even local antibiotic administration could be applied.
c) Diagnosis and clarification of etiology of PE depends on volume and location
Of note is the fact that small effusions (50 to 100 mL) are only seen posteriorly, with typically less than 10 mm in thickness, and only cause minimal separation between the epicardial (visceral) pericardium and the thicker parietal pericardial sac. As an EBUS in the distal left main bronchus is positioned at the posterolateral wall of the LV it can be assumed that the early diagnosis could be easier performed with EBUS (similar to transesophagial echocardiography) than with transthoracic ultrasound. The same consideration could be true for located PE by septation. In series of patients with acute pericarditis (including those with small or undetectable effusions), the underlying diagnosis was established in only about 15 to 20 percent.51,52 In contrast, studies evaluating moderate to large pericardial effusions have revealed diagnoses in up to 90 percent of cases when very aggressive diagnostic approaches are taken.53 Such aggressive approaches are often not warranted in clinical practice, however, given the benign natural history of many of these effusions. It was illustrated in a review of 322 patients with a moderate or large pericardial effusion54 that in 60 percent of patients, the cause of the effusion was a known medical condition. Therefore, any intervention for clarification of the etiology has to be outweighed on the background of the medical available information. However; especially in patients with known malignancy the rationale that the diagnosis of the medical condition is the same diagnosis of the pericardial effusion is not always true as explained below.
d) Therapeutic aspiration should be applied in any case of even moderate PE if the risk of such a new procedure outweighs the physiologic benefits of near normal PE due to improved hemodynamics
Experimental 55-57 and clinical studies23,58 have shown that cardiac tamponade is not a 'black or white' phenomenon: It is a continuum that goes from slight elevations of intrapericardial pressure with subtle hemodynamic repercussion to a situation of severe hemodynamic perturbation until death. The concept of continuum was elegantly illustrated by Reddy et al. 23 that even subtle elevations of intrapericardial pressure by small or moderate PE have hemodynamic consequences. He concluded that the severity of hemodynamic derangement rather than the presence or absence should be assessed in patients with PE. This aspect is an important consideration as any PE would change hemodynamics which is one of the most important parameters to sustain life. Therefore, a (new) technique easy to apply to reduce even moderate PE would make sense in some clinically overt heart insufficiency situations. Moreover, correct assessment of pulmonary hypertension (PH) is only possible if sometimes existing (even moderate) PE can be reduced to the lowest possible volume as to diminish the interference of PH and PE. This is especially important in collagen vascular disease which was true in one of the hereby shown 2 cases of Sjögren's disease with pericardial cytology specimen considered to be a lymphoma in the first examination (Fig. 19). Originally this patient was sent to evaluate possible pulmonary arterial hypertension as part of the known Sjögren's syndrome. Both diagnosis - PH and lymphoma - could be ruled out. However; it is noteworthy that a Sjögren's syndrome with these comorbidities has a much poorer prognosis than without.59-61
A chronical large PEs in a low pressure tamponade scenario can convert suddenly without any pre-occasional sign into a life-threatening classical tamponade, therefore it is recommended to attempt to reduce the large volume.62 Very little fluid needs to accumulate to produce cardiac tamponade once the pericardium can no longer stretch26 which is more probable in chronical large effusions than in smaller ones although individual factors as explained above leads to a highly individual threshold. At this point, the initial removal of fluid during pericardiocentesis produces the largest reduction in intrapericardial pressure and shows therefore the greatest hemodynamic release. As an alternative to a pericardiocentesis we are sure that the hereby first reported method of TPEA could be easily used in these scenarios.
Edited LLPV, PE, RCX, LA in different depth scales, rotations and bw/CPA. LLPV=Left lower pulmonary vein. PE=Pericardial effusion. RCX= Circumflex coronary artery. LA=Left atrium.
e) Effusive constrictive pericarditis (ECP)
ECP is characterized by underlying constrictive physiology with a coexisting pericardial effusion, usually / often with cardiac tamponade. Such patients may be mistakenly thought to have only PE up to cardiac tamponade; however, elevation of the right atrial and pulmonary wedge pressures after drainage of the pericardial fluid points to the underlying constrictive process. An important pathophysiologic feature of both cardiac tamponade and constrictive pericarditis (CP) is greatly enhanced by ventricular interaction or interdependence, in which the hemodynamics of the left and right heart chambers are directly influenced by each other to a much greater degree than normal.63 ECP has been best characterized in patients with tamponade who continue to have elevated intracardiac pressure after the removal of pericardial fluid mostly by pericardiocentesis. In these scenarios simultaneous intrapericardial and hemodynamic pressure measurements are the gold standard of diagnostic approach allowing the exact diagnosis of ECP. There are only few studies applying such relatively aggressive approach. Of note is the fact that intrapericardial pressure measurements with TPEA is possible by using regular pressure transducers at the end of the EBUS needle - we have performed such approach in two patients already for monitoring purposes. ECP is due to pericardial inflammation (or (mostly malignant) infiltration) causing constriction in conjunction with the presence of pericardial fluid under pressure. The etiology is diverse with similar causes to CP and the condition is more prevalent with certain etiologies such as tuberculous, purulent and malignant pericarditis. However, theoretically any PE can lead to CP - we simply do not know the clinical trait of a so called idiopathic PE in each individual patient. It is unclear if reduction of initial moderate volume of a first PE in ECP reduces the rate of CP in future in an individual scenario. As inflammation is believed to be the cornerstone of the development of CP it has to be mentioned that effusion is not only a sign but as well a medium of an inflammatory disease: As medium an effusion has the function of transporting inflammatory properties towards the visceral leaf of the pericardial sac resulting into CP. In that context of interesting note is the rarely described fact that contents of the pericardial fluid regulate the coronary arterial tone.22 This is why we believe that with a (so far) easy and unrisky method to aspirate moderate PE for reducing these intrapericardial inflammatory properties especially in e.g. immun disorders one should apply such a method more frequent. As shown below in immun disorders the risk of developing CP is six times higher than in idiopathic / viral PE.64-70
One of the most relevant publications looking for the development of CP after a first PE revealed the following statistics:
During a median follow-up of 72 months of 500 patients between 2000 and 2008, CP developed in only 1.8% of the patients: 2 of 416 patients with idiopathic/viral pericarditis (0.48%) versus 7 of 84 patients with a nonviral/nonidiopathic etiology (8.3%). The incidence rate of CP per 1000 person-years was 0.76 cases for idiopathic/viral pericarditis, 4.40 cases for connective tissue disease/pericardial injury syndrome, 6.33 cases for neoplastic pericarditis, 31.65 cases for tuberculous pericarditis, and 52.74 cases for purulent pericarditis.64 Of note is the fact that CP does not always show signs of increased thickness of pericardium: Talreja showed in a surgical series with histolgy proven CP in 143 patients that 18% of these definite cases did not show an enlarged thickness.71 In a review Ntsekhe reported that the pericardiectomy rate is around 65% in all diagnosed patients with ECP.72 Therefore a significant number of patients with ECP / CP will require pericardiectomy with periprocedural 30-d mortality rates of around 20%.73-75
A proportion of patients have a predominantly inflammatory and reversible pericardial reaction and may improve with the treatment of the underlying cause and the use of anti-inflammatory medications which may work better if applied directly intrapericardially (possibly performed by TPEA) as mentioned above in uremic pericarditis with PE. In regards to this high mortality rate of the often essential surgical therapy all means to reduce the probability of a developing CP as the final form of a progressing ECP should be applied. TPEA could be easily used beside uremic, purulent or malignant PEs especially in PEs caused by immune disorders. This aspect of beneficial aspiration of PE in immune disorders due to the higher risk of developing ECP is so far not included in the actual guidelines. For us it was one aspect to apply TPEA in the 2 Sjögren's syndromes with PE in this series.
II) Therapeutic considerations
Beside the above-mentioned situations when intrapericardial therapy was applied and seemed to be beneficial TPEA clearly could be a therapeutical approach especially in malignant PE: One of the major applications of EBUS is transbronchial needle aspiration of malignant lymph nodes. It is well known that intrapericardial application of chemotherapy is beneficial for the prognosis and quality of life of patients with malignant PE.76-80 As the EBUS-TBNA needle has an inner stylet the needle can be used to administer chemotherapy which has already been done in malignant enlarged lymph nodes as mentioned above.15,16,81 It is logical to assume that we could apply with TPEA in the same mode for malignant PEs with good results. The management of cardiac tamponade (or large PE) in patients with neoplastic pericardial involvement merits a special comment. Simple pericardiocentesis alleviates symptoms in most cases but PE relapses in as many as 40-50% of patients. 82 Therefore we expect that TPEA as a comparable alternative in these patients would meet its goal in around half of these patients with the first (and only) attempt. Indwelling pericardial catheters over days have a success rate (defined as alleviation of tamponade and no need of further procedures) of 75% approximately.62 The catheter should be removed when the amount of drainage is lower than 25 mL/d. In different series the duration of catheter drainage averaged 4.8 d.83-86_ Catheter infection is a very rare complication. Due to this knowledge we expect that TPEA has only a theoretical risk of infection when performed along this hereby first described algorithm. The aims of a prolonged indwelling pericardial catheter are to achieve a complete pericardial drainage and to provoke adherence between the two layers of the pericardium in order to prevent recurrence of PE. This goal can be favored by intrapericardial sclerosis with tetracycline or other agents. Taken TPEA as an alternative even in a recurrent malignant PE it is assumable that we can apply these sclerosing agents instead of second line pericardiocentesis with these agents. However, some authors have observed no additional advantages over indwelling pericardial catheters and sclerosing agents can provoke 'excessive' sclerosis with evolution to constrictive pericarditis with clinical repercussion.87 Balloon pericardiotomy has been adopted for patients with malignancy and reduced life expectancy, and it is successful in more than 80% of cases.88-91 Reported complications include fever, pneumothorax, left pleural effusion and bleeding from pericardial blood vessels.90,91 Especially the pneumothorax risk could be avoided by TPEA. Surgical drainage procedures with different complexity have a higher efficacy in relieving PE (80%-90%).92 Perioperative risks, especially if performed under general anesthesia, are a concern. As explained above we never expected to use general anesthesia in TPEA. The overall 30 d mortality for surgical drainage of malignant PE has been reported to be around 20% .73-75 In a multivariate analysis in 423 patients referred for symptoms of CP about the development of the lethal Post Pericardiotomy Low Cardiac Output Syndrome (POLCOS) Sabzi et al.62 reported about the following significant odds ratios (OR) for death: Malignancy 1,12; radiotherapy 2,01; calcification of pericardium 1,78 and preoperative (low) ejection fraction 2,06. CP with an OR of 3,55 was not significant in this multivariate analysis (p= 0,055). In contrary in univariate analysis CP showed the highest significant OR of 4,67 (p=0,006). Some patients with malignant PE demonstrate persistence of clinical findings of systemic venous hypertension after effective drainage of the PE. In these cases, an effusive-constrictive pericarditis should be considered even in malignancies.93 In a recent systematic review of Virk et al. 94 looking for recurrence after minimal-invasive intervention isolated pericardiocentesis demonstrated a pooled recurrence rate of 38.3%. Pooled recurrence rates for extended catheter drainage, pericardial sclerosis with one pericardiocentesis and percutaneous balloon pericardiotomy were 12.1%, 10.8% and 10.3%, respectively. Procedure-related mortality ranged from 0.5-1.0% across the percutaneous catheter or balloning interventions.95 As mentioned above we believe therefore that TPEA second line with instillation of sclerosing agents is a feasible alternative to pericardiocentesis with sclerosing agents when a first PE drainage leads to a recurrence. Although having performed so far only 10 procedures we believe that TPEA is a considerably safe approach for first and second line treatment.
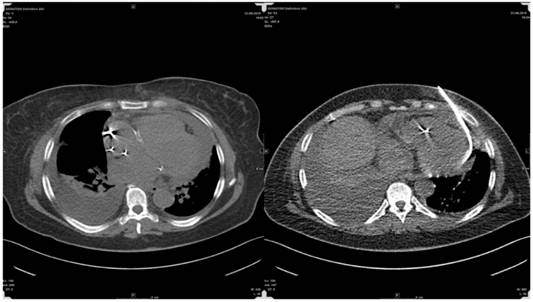
The finding of a PE in patients with underlying malignancy creates a more complex dilemma, as not infrequently PE is due to alternative causes and not to direct neoplastic pericardial involvement. Posner et al.96 diagnosed in his series malignant pericardial disease in 18 (58%) of 31 patients with underlying cancer and pericarditis with pericardial effusion, while 32% of the patients had idiopathic pericarditis and 10% had radiation induced pericarditis. Porte et al. 97 studied 114 patients with recent or remote history of cancer and a PE of unknown origin requiring drainage for diagnostic or therapeutic purposes. Pericardioscopy was performed in 112 patients with pericardial fluid analysis and biopsy of abnormal structures or deposits under direct visual control. Malignant pericardial disease was found in 44 (38%) patients, while 70 (61%) patients had non-malignant pericardial effusions (idiopathic in 33 patients, radiation-induced in 20 patients, infectious effusion in 10 patients, and hemopericardium as a result of coagulation disorders in 8 patients). These studies show that, in more than half of the patients with underlying (present or recent even remote non-thoracical) cancer, a PE is due to causes different than direct neoplastic involvement. Therefore, the precise etiology of these PEs needs to be clarified due to obvious prognostic and therapeutic consequences. We believe that TPEA is a comparable easy and feasible method to perform such clarification especially in patients with actual or former malignant disease as we did in this series. Of importance is the fact that some oncological drugs may induce PE as well. Tyrosine kinase inhibitors often used in palliative situations in adenocarcinomas of the lung and doxorubicin can cause a special confusion in these patients as well.98 It has to be mentioned that beside interventional cardiologists and pulmonologists transthoracic approaches for PE are as well applied by interventional radiologists with high expertise: Following a personal note of Thomas Vogl the drainage of large PEs under CT-guidance is an easy, quick and safe approach. He reported about 150 drainages without any problems - yet approaching the pericardial sac antior.(Fig. 20) Another young field in interventional minimal-invasive medicine is epicardial ablation treatments for complex scars refractory to endocardial ablation (especially due to Chagas or ischemic heart disease) and various arrhythmogenic substrates by entering the ablation-catheters percutaneously into the pericardial sac.99-101 However this technique is not wide spread even in bigger Electrophysiology (EP) centres as the approach with a so called blind tap comprises a high risk of major complications including death up to 4-5% with a minor complication rate of 30% including pericarditis and PE.102,103 Delayed PE after endocardial ablation like in pulmonary vein isolation has occured.104,105 Interestingly recent experimental publications on an 'easy' approach for pulmonary vein isolation in atrial fibrillation consider transapical puncture of the left ventricle for retrograde transmitral access to the pulmonary veins.106
In regards to such a proposed and in our view 'aggressive' approach it is quite assumable that - as already mentioned19 - with TPEA even perfused vascular or cardiac structures are easily entered in a very controllable manner. Possibly TPEA giving a new window to the heart will play a role in this kind of approach one day. We already performed one monopolar mapping through the Olympus EBUS-needle without any problem on the epicardial surface.
Radiologists' approach for pericardial effusion aspiration.
In summary: TPEA along our proposed algorithm seems to be a safe and easy new window to the pericardial sac which can be used for diagnostics and treatments of pericardial and other cardiac diseases.
Competing Interests
None to declare.
References
1. Domvri K, Zarogoulidis P, Darwiche K. et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. Journal of Cancer. 2013;4(9):736-754
2. Baltayiannis N, Chandrinos M, Anagnostopoulos D. et al. Lung cancer surgery: an up to date. Journal of thoracic disease. 2013;5(Suppl 4):S425-439
3. Zarogoulidis K, Zarogoulidis P, Darwiche K. et al. Treatment of non-small cell lung cancer (NSCLC). Journal of thoracic disease. 2013;5(Suppl 4):S389-396
4. Domvri K, Darwiche K, Zarogoulidis P, Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Translational lung cancer research. 2013;2(4):256-258
5. Milovancev A, Stojsic V, Zaric B. et al. EGFR-TKIs in adjuvant treatment of lung cancer: to give or not to give? OncoTargets and therapy. 2015;8:2915-2921
6. Lampaki S, Lazaridis G, Zarogoulidis K. et al. Defining the role of tyrosine kinase inhibitors in early stage non-small cell lung cancer. Journal of Cancer. 2015;6(6):568-574
7. Papadopoulou E, Tsoulos N, Tsirigoti A. et al. Determination of and mutational status in Greek non-small-cell lung cancer patients. Oncology letters. 2015;10(4):2176-2184
8. Gompelmann D, Eberhardt R, Herth FJ. Interventional pulmonology procedures: an update. Panminerva medica. 2013;55(2):121-129
9. Wahidi MM, Herth F, Yasufuku K. et al. Technical Aspects of Endobronchial Ultrasound Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest. 2015
10. Yarmus LB, Akulian J, Lechtzin N. et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest. 2013;143(4):1036-1043
11. Xiong S, Liu N, Han Q, Jiang Y, Su X. [Diagnostic value of endobronchial ultrasound-guided transbronchial needle aspiration combined with Surepath liquid-based cytology test for lung lesions and mediastinal lymphadenopathy]. Zhonghua bing li xue za zhi Chinese journal of pathology. 2015;44(9):633-638
12. Harris RM, Arnaout R, Koziel H, Folch E, Majid A, Kirby JE. Utility of microbiological testing of thoracic lymph nodes sampled by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in patients with mediastinal lymphadenopathy. Diagnostic microbiology and infectious disease. 2015
13. Oezkan F, Khan A, Zarogoulidis P. et al. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. OncoTargets and therapy. 2014;7:2061-2065
14. Hohenforst-Schmidt W, Petermann A, Visouli A. et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug design, development and therapy. 2013;7:627-633
15. Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K. et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug design, development and therapy. 2013;7:571-583
16. Mehta HJ, Begnaud A, Penley AM. et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung cancer. Dec. 2015;90(3):542-547
17. Park JS, Chung JH, Jheon S. et al. EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. The European respiratory journal. 2011;38(6):1480-1482
18. Goyal R, Chachra V, Gogia P. Diagnosis of pulmonary embolism by endobronchial ultrasound. Lung India: official organ of Indian Chest Society. 2015;32(6):606-608
19. He MC. Dr. Paul Zarogoulidis: the exploration on pneumothorax and new use of EBUS. Journal of thoracic disease. 2015;7(Suppl 1):S73-74
20. Hohenforst-Schmidt W, Banckwitz R, Zarogoulidis P. et al. Radiation Exposure of Patients by Cone Beam CT during Endobronchial Navigation - A Phantom Study. Journal of Cancer. 2014;5(3):192-202
21. Sagrista-Sauleda J, Angel J, Sambola A, Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation. 2008;117(12):1545-1549
22. Miyazaki T, Pride HP, Zipes DP. Prostaglandins in the pericardial fluid modulate neural regulation of cardiac electrophysiological properties. Circulation research. 1990;66(1):163-175
23. Reddy PS, Curtiss EI, Uretsky BF. Spectrum of hemodynamic changes in cardiac tamponade. The American journal of cardiology. 1990;66(20):1487-1491
24. Sagrista-Sauleda J, Angel J, Sambola A, Alguersuari J, Permanyer-Miralda G, Soler-Soler J. Low-pressure cardiac tamponade: clinical and hemodynamic profile. Circulation. 2006;114(9):945-952
25. Reddy PS, Curtiss EI, O'Toole JD, Shaver JA. Cardiac tamponade: hemodynamic observations in man. Circulation. 1978;58(2):265-272
26. Spodick DH. Acute cardiac tamponade. The New England journal of medicine. 2003;349(7):684-690
27. Yarmus L, Akulian J, Ortiz R. et al. A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. Journal of thoracic disease. 2015;7(10):1825-1832
28. Kraincuk P, Kormoczi G, Prokop M, Ihra G, Aloy A. Alveolar recruitment of atelectasis under combined high-frequency jet ventilation: a computed tomography study. Intensive care medicine. 2003;29(8):1265-1272
29. Ihra G, Kepka A, Lanzenberger E. et al. [SHFJV. Jet-adapter for application of superimposed high-frequency jet ventilation (SHFJV) via a tube in intensive care: a new technique]. Der Anaesthesist. 1998;47(3):209-219
30. Chung KK, Wolf SE, Renz EM. et al. High-frequency percussive ventilation and low tidal volume ventilation in burns: a randomized controlled trial. Critical care medicine. 2010;38(10):1970-1977
31. Schragl E, Donner A, Kashanipour A, Aloy A. [Preliminary experiences with superimpoposed high-frequence jet ventilation in intensive care]. Der Anaesthesist. 1995;44(6):429-435
32. Schragl E, Donner A, Kashanipour A, Ullrich R, Aloy A. [Superimposed high-frequency jet ventilation (SHFJV) in the administration of NO. Technical basis and early clinical results with ARDS]. Der Anaesthesist. 1995;44(12):843-849
33. Maisch B, Rupp H, Ristic A, Pankuweit S. Pericardioscopy and epi- and pericardial biopsy - a new window to the heart improving etiological diagnoses and permitting targeted intrapericardial therapy. Heart failure reviews. 2013;18(3):317-328
34. Authors/Task Force M, Adler Y, Charron P. et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). European heart journal. 2015;36(42):2921-2964
35. Imazio M. Idiopathic recurrent pericarditis as an immune-mediated disease: current insights into pathogenesis and emerging treatment options. Expert review of clinical immunology. 2014;10(11):1487-1492
36. Imazio M, Brucato A, Barbieri A. et al. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation. 2013;128(1):42-49
37. Imazio M, Belli R, Beqaraj F. et al. DRainage Or Pericardiocentesis alone for recurrent nonmalignant, nonbacterial pericardial effusions requiring intervention: rationale and design of the DROP trial, a randomized, open-label, multicenter study. Journal of cardiovascular medicine. 2014;15(6):510-514
38. Sadjadi SA, Mashahdian A. Uremic pericarditis: a report of 30 cases and review of the literature. The American journal of case reports. 2015;16:169-173
39. De Pace NL, Nestico PF, Schwartz AB. et al. Predicting success of intensive dialysis in the treatment of uremic pericarditis. The American journal of medicine. 1984;76(1):38-46
40. Buselmeier TJ, Davin TD, Simmons RL, Najarian JS, Kjellstrand CM. Treatment of intractable uremic pericardial effusion. Avoidance of pericardiectomy with local steroid instillation. Jama. 1978;240(13):1358-1359
41. Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Annals of internal medicine. 1972;77(4):507-513
42. Sagrista-Sauleda J, Barrabes JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. Journal of the American College of Cardiology. 1993;22(6):1661-1665
43. Ferreira dos Santos L, Moreira D, Ribeiro P. et al. [Purulent pericarditis: a rare diagnosis]. Revista portuguesa de cardiologia: orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology: an official journal of the Portuguese Society of Cardiology. 2013;32(9):721-727
44. Leoncini G, Iurilli L, Queirolo A, Catrambone G. Primary and secondary purulent pericarditis in otherwise healthy adults. Interactive cardiovascular and thoracic surgery. 2006;5(5):652-654
45. Goodman LJ. Purulent Pericarditis. Current treatment options in cardiovascular medicine. 2000;2(4):343-350
46. Magishi K, Izumi Y, Shimizu N. [Rapid transition from purulent to constrictive pericarditis]. Kyobu geka. The Japanese journal of thoracic surgery. 2014;67(6):463-466
47. Gundelly P, Thornton A, Greenberg RN, McCormick M, Myint T. Rhodococcus equi pericarditis in a patient living with HIV/AIDS. Journal of the International Association of Providers of AIDS Care. 2014;13(4):309-312
48. Cruz D, Ahmed H, Gandapur Y, Abraham MR. Propionibacterium acnes: A Treatable Cause of Constrictive Pericarditis. Case reports in medicine. 2015;2015:193272
49. Sotoudeh Anvari M, Kianinejad R, Boroumand MA, Arzhan S, Jalali A. Bacterial pericarditis and antimicrobial resistance at the Tehran Heart Center, Iran. Journal of infection in developing countries. 2015;9(7):780-784
50. Cilloniz C, Rangel E, Barlascini C, Piroddi IM, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series. Jornal brasileiro de pneumologia: publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2015;41(4):389-394
51. Permanyer-Miralda G, Sagrista-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. The American journal of cardiology. 1985;56(10):623-630
52. Zayas R, Anguita M, Torres F. et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. The American journal of cardiology. 1995;75(5):378-382
53. Corey GR, Campbell PT, Van Trigt P. et al. Etiology of large pericardial effusions. The American journal of medicine. 1993;95(2):209-213
54. Sagrista-Sauleda J, Merce J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. The American journal of medicine. 2000;109(2):95-101
55. Leimgruber PP, Klopfenstein HS, Wann LS, Brooks HL. The hemodynamic derangement associated with right ventricular diastolic collapse in cardiac tamponade: an experimental echocardiographic study. Circulation. 1983;68(3):612-620
56. Klopfenstein HS, Cogswell TL, Bernath GA. et al. Alterations in intravascular volume affect the relation between right ventricular diastolic collapse and the hemodynamic severity of cardiac tamponade. Journal of the American College of Cardiology. 1985;6(5):1057-1063
57. Gonzalez MS, Basnight MA, Appleton CP. Experimental pericardial effusion: relation of abnormal respiratory variation in mitral flow velocity to hemodynamics and diastolic right heart collapse. Journal of the American College of Cardiology. 1991;17(1):239-248
58. Singh S, Wann LS, Schuchard GH. et al. Right ventricular and right atrial collapse in patients with cardiac tamponade-a combined echocardiographic and hemodynamic study. Circulation. 1984;70(6):966-971
59. Kokosi M, Riemer EC, Highland KB. Pulmonary involvement in Sjogren syndrome. Clinics in chest medicine. 2010;31(3):489-500
60. Lin DF, Yan SM, Zhao Y. et al. Clinical and prognostic characteristics of 573 cases of primary Sjogren's syndrome. Chinese medical journal. 2010;123(22):3252-3257
61. Rajani AR, Hussain K, Baslaib FO, Rao KN. An unexpected diagnosis in a dyspnoeic patient with primary Sjogren syndrome. BMJ case reports. 2013:2013
62. Sagrista-Sauleda J, Merce AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World journal of cardiology. 2011;3(5):135-143
63. Syed FF, Ntsekhe M, Mayosi BM, Oh JK. Effusive-constrictive pericarditis. Heart failure reviews. 2013;18(3):277-287
64. Imazio M, Brucato A, Maestroni S. et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124(11):1270-1275
65. LeWinter MM. Clinical practice. Acute pericarditis. The New England journal of medicine. Dec 18. 2014;371(25):2410-2416
66. Imazio M, Gaita F. Diagnosis and treatment of pericarditis. Heart. 2015;101(14):1159-1168
67. Imazio M. Contemporary management of pericardial diseases. Current opinion in cardiology. 2012;27(3):308-317
68. Cho IJ, Chang HJ, Chung H. et al. Differential Impact of Constrictive Physiology after Pericardiocentesis in Malignancy Patients with Pericardial Effusion. PloS one. 2015;10(12):e0145461
69. Han Z, Li S, Jing H, Liu H. Primary idiopathic chylopericardium: a retrospective case series. BMC surgery. 2015;15:61
70. Shivamurthy P, Brar N, Therrien ML. Isolated hemopericardium associated with rivaroxaban: first case report. Pharmacotherapy. 2014;34(9):e169-172
71. Talreja DR, Edwards WD, Danielson GK. et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108(15):1852-1857
72. Ntsekhe M, Shey Wiysonge C, Commerford PJ, Mayosi BM. The prevalence and outcome of effusive constrictive pericarditis: a systematic review of the literature. Cardiovascular journal of Africa. 2012;23(5):281-285
73. Piehler JM, Pluth JR, Schaff HV, Danielson GK, Orszulak TA, Puga FJ. Surgical management of effusive pericardial disease. Influence of extent of pericardial resection on clinical course. The Journal of thoracic and cardiovascular surgery. 1985;90(4):506-516
74. Busch C, Penov K, Amorim PA. et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2015;48(6):e110-116
75. Bicer M, Ozdemir B, Kan I, Yuksel A, Tok M, Senkaya I. Long-term outcomes of pericardiectomy for constrictive pericarditis. Journal of cardiothoracic surgery. 2015;10(1):177
76. Ji YL, Li RZ, Xue LF. et al. Therapeutic effects of 5-fluorouracil sustained-release particles in 81 malignant pericardial effusion patients. The Kaohsiung journal of medical sciences. 2015;31(2):96-101
77. Martinoni A, Cipolla CM, Civelli M. et al. Intrapericardial treatment of neoplastic pericardial effusions. Herz. 2000;25(8):787-793
78. Tomkowski WZ, Filipecki S. Intrapericardial administration of cisplatin in treatment of metastatic pericardial involvement in adenocarcinoma of the lung. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace / Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo. 1997;52(3):221-224
79. Tomkowski WZ, Wisniewska J, Szturmowicz M. et al. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2004;12(1):53-57
80. Lestuzzi C, Berretta M, Tomkowski W. 2015 update on the diagnosis and management of neoplastic pericardial disease. Expert review of cardiovascular therapy. 2015;13(4):377-389
81. Khan F, Anker CJ, Garrison G, Kinsey CM. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Annals of the American Thoracic Society. 2015;12(1):101-104
82. Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. Jama. 1994;272(1):59-64
83. Lock JE, Bass JL, Kulik TJ, Fuhrman BP. Chronic percutaneous pericardial drainage with modified pigtail catheters in children. The American journal of cardiology. 1984;53(8):1179-1182
84. Wei JY, Taylor GJ, Achuff SC. Recurrent cardiac tamponade and large pericardial effusions: management with an indwelling pericardial catheter. The American journal of cardiology. 1978;42(2):281-282
85. Kopecky SL, Callahan JA, Tajik AJ, Seward JB. Percutaneous pericardial catheter drainage: report of 42 consecutive cases. The American journal of cardiology. 1986;58(7):633-635
86. Patel AK, Kosolcharoen PK, Nallasivan M, Kroncke GM, Thomsen JH. Catheter drainage of the pericardium. Practical method to maintain long-term patency. Chest. Dec. 1987;92(6):1018-1021
87. The pericardium. A Comprehensive Textbook. New York: Marcel Dekker. 1997
88. Palacios IF, Tuzcu EM, Ziskind AA, Younger J, Block PC. Percutaneous balloon pericardial window for patients with malignant pericardial effusion and tamponade. Catheterization and cardiovascular diagnosis. 1991;22(4):244-249
89. Ziskind AA, Pearce AC, Lemmon CC. et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. Journal of the American College of Cardiology. 1993;21(1):1-5
90. Wang HJ, Hsu KL, Chiang FT, Tseng CD, Tseng YZ, Liau CS. Technical and prognostic outcomes of double-balloon pericardiotomy for large malignancy-related pericardial effusions. Chest. 2002;122(3):893-899
91. Swanson N, Mirza I, Wijesinghe N, Devlin G. Primary percutaneous balloon pericardiotomy for malignant pericardial effusion. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2008;71(4):504-507
92. Sabzi F, Faraji R. Predictors of post pericardiotomy low cardiac output syndrome in patients with pericardial effusion. Journal of cardiovascular and thoracic research. 2015;7(1):18-23
93. Sagrista-Sauleda J, Angel J, Sanchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. The New England journal of medicine. 2004;350(5):469-475
94. Virk SA, Chandrakumar D, Villanueva C, Wolfenden H, Liou K, Cao C. Systematic review of percutaneous interventions for malignant pericardial effusion. Heart. 2015;101(20):1619-1626
95. Kumar R, Sinha A, Lin MJ. et al. Complications of pericardiocentesis: A clinical synopsis. International journal of critical illness and injury science. 2015;5(3):206-212
96. Posner MR, Cohen GI, Skarin AT. Pericardial disease in patients with cancer. The differentiation of malignant from idiopathic and radiation-induced pericarditis. The American journal of medicine. 1981;71(3):407-413
97. Porte HL, Janecki-Delebecq TJ, Finzi L, Metois DG, Millaire A, Wurtz AJ. Pericardoscopy for primary management of pericardial effusion in cancer patients. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 1999;16(3):287-291
98. Agrawal V, Christenson ES, Showel MM. Tyrosine kinase inhibitor induced isolated pericardial effusion. Case reports in oncology. 2015;8(1):88-93
99. Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. Journal of cardiovascular electrophysiology. 1996;7(6):531-536
100. Sosa E, Scanavacca M, D'Avila A, Bellotti G, Pilleggi F. Radiofrequency catheter ablation of ventricular tachycardia guided by nonsurgical epicardial mapping in chronic Chagasic heart disease. Pacing and clinical electrophysiology: PACE. 1999;22(1 Pt 1):128-130
101. Sosa E, Scanavacca M, d'Avila A, Tondato F, Kunyoshi R, Elias J. Nonsurgical transthoracic mapping and ablation in a child with incessant ventricular tachycardia. Journal of cardiovascular electrophysiology. 2000;11(2):208-210
102. Della Bella P, Brugada J, Zeppenfeld K. et al. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circulation. Arrhythmia and electrophysiology. 2011;4(5):653-659
103. Sacher F, Roberts-Thomson K, Maury P. et al. Epicardial ventricular tachycardia ablation a multicenter safety study. Journal of the American College of Cardiology. 2010;55(21):2366-2372
104. Goossens K, Caenepeel A, De Greef Y. Delayed tamponade triggering Dressler's syndrome after pulmonary vein isolation. Acta cardiologica. 2012;67(5):595-598
105. Torihashi S, Shiraishi H, Hamaoka T. et al. Two cases of delayed cardiac tamponade due to pericarditis after pulmonary vein (PV) isolation for atrial fibrillation. Internal medicine. 2015;54(7):791-796
106. Bollmann A, Kosiuk J, Hilbert S, John S, Hindricks G. Percutaneous transapical access for pulmonary vein mapping and ablation in a porcine model with a new high-density electroanatomical mapping system. International journal of clinical and experimental medicine. 2015;8(8):12631-12636
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph. D. Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece. Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom.
Corresponding author: Paul Zarogoulidis, M.D, Ph. D. Pulmonary Department-Oncology Unit, “G. Papanikolaou” General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece. Fax: 00302310992424 Mobile: 00306977271974 E-mail: pzarogcom.