J Biomed 2017; 2:57-63. doi:10.7150/jbm.18881 This volume Cite
Research Paper
Candesartan suppresses proteinuria and decrease of nephrin expression but hydralazine does not in hypertensive nephropathy
1. Department of Pharmacology, Faculty of Medicine, Kindai University, Osaka, Japan;
2. Department of Pathology, Faculty of Medicine, Kindai University, Osaka, Japan;
3. Life Science Institute, Kindai University, Osaka, Japan.
Received 2016-12-22; Accepted 2017-2-1; Published 2017-2-23
Abstract
Proteinuria is considered as an indicator of glomerular podocyte injuries and a major cause of chronic kidney disease. Podocytes include important and functional proteins including nephrin. We previously reported proteinuria and nephrin loss in the podocytes of malignant stroke-prone spontaneous hypertensive rats (MSHRSPs), a hypertensive nephropathy model. At first, we actually confirmed that the nephrin was reduced in the human glomeruli with diabetic nephropathy by immunohistochemistry. These specimens showed the tendency of increased blood pressure. Moreover, to investigate the molecular mechanisms of proteinuria and podocyte injuries, we examined the effects of a vasodilator, hydralazine, and an angiotensin receptor blocker, candesartan hydrochloride on the proteinuria and nephrin expression in MSHRSPs. As results, hydralazine had preventive effects on tubular injuries and renal dysfunction but not on podocyte injuries because hydralazine-treated MSHR developed proteinuria and exhibited nephrin loss from the glomeruli. However, candesartan plays protective functions against proteinuria and the decrease of nephrin in addition to the tubular injuries. Thus, candesartan could significantly suppress podocyte injuries. We conclude that podocyte injuries and proteinuria were dependent on an excessive angiotensin system, not mechanical stress.
Keywords: angiotensin receptor blocker, diabetic nephropathy, hypertensive nephropathy, nephrin, podocyte, vasodilator.
Introduction
When blood plasma passes the glomerular capillary loops, the local intracapillary pressure drives plasma through the glomerular filtration barrier that consists of three layers: podocytes, the basement membrane, and endothelial cells [1]. Proteinuria due to damages of this barrier is an indicator of kidney diseases and is largely caused by glomerular diseases, such as diabetes or glomerulonephritis [2]. Podocytes play a central barrier role for the urine, and their injury leads to the nephropathies [3,4]. The glomerular slit diaphragm (SD) serves as a size-selective barrier and it is linked to the actin-based cytoskeleton by adaptor proteins [5, 6]. Nephrin is expressed in podocytes, and a transmembrane protein of the immunoglobulin family of cell-adhesion molecules, and localized to the SD. A mutational inactivation of nephrin leads into congenital nephrotic syndrome of the Finnish type [7], and nephrin knockout mice show effacement of podocyte foot process and absence of SD and massive proteinuria [8]. Thus, nephrin is necessary for podocytes to prevent the blood proteins leaking into urine and important for life maintenance.
The epidemiological data from dialysis registries has shown that the incidence of end-stage renal disease (ESRD) is progressively increasing in Japan and other countries. Diabetic nephropathy is one of the most serious microvascular complications in diabetes and the leading cause of ESRD [9]. Especially, hypertension is twice as prevalent in patients with diabetes compared to the general population with mean BP rising by 5-8% a year in those with nephropathy [10]. Therefore, new strategies for the prevention and treatment of renal diseases involving hypertension are urgently required. Recently, we showed that the pathophysiology of malignant SHRSP/Kpo (MSHRSP); they exhibited a higher blood pressure (BP) at 6 weeks on comparison with WKY/Kpo (WKY) [11]. A pathological characteristic of this strain is hypertensive nephropathy with proteinuria and renal dysfunction, and they showed the glomerular endothelial hypertrophy and podocyte injuries. Notably, nephrin and other podocyte's proteins were lost in the MSHRSP glomeruli [11]. It is here important to confirm the nephrin loss in human glomeruli with DN, and we performed the immunohistochemical assay for nephrin.
Moreover, to search for a most effective therapy for nephropathies with hypertension, we focused on renin-angiotensin (Ang) system (RAS). The RAS plays a central role in the control of the systemic BP. The use of RAS inhibitors, such as Ang-converting enzyme inhibitors and Ang receptor blockers (ARBs), is effective for inhibiting the progression of established renal diseases including hypertensive nephropathy [12-14]. Furthermore, we investigated preventive effects of anti-hypertension drugs, a vasodilator, hydralazine, and an ARB, candesartan hydrochloride focusing on podocyte injuries and proteinuria of hypertensive nephropathies.
Materials and Methods
Analysis of human nephropathy
We received clinical records, autopsy records, and pathological specimens archived in Kindai University Hospital (Osaka, Japan) to identify autopsied patients who had been diagnosed as diabetic nephropathy. For controls, we selected patients without clinical and histological evidence of kidney involvements. Specimens were fixed with 10%-buffered formalin, embedded in paraffin, and cut into 4-μm sections. The ethics committee of Kindai University Faculty of Medicine approved the experimental protocol and waived the need for written informed consent (26-279). We performed E-cadherin immunohistochemistry into the sections and excluded the specimens with less or no staining of that before analyses due to excessive autolysis. Systolic BP could not be chased against some specimens, in which nephrin level was measured.
Animal experiment
We maintained MSHRSP and WKY rats and measured BP as described previously [15]. All animal experiments were carried out according to the Guideline for Experimental Animal Care issued by the Prime Minister's Office of Japan and approved by the Committee on Animal Experimentation of Kindai University School of Medicine. SDS-PAGE analysis of urine was performed as described previously [15].
Histological analysis and nephrin expression
Right kidney removed in MSHRSP at each autopsy was fixed in cold 10%-buffered formalin for 24 h respectively. Transversally trimmed kidney tissues were submitted to a routine process for paraffin embedding [16, 17]. Anti-nephrin IgG (sc-19000, Santa Cruz Biotechnology, Dallas, TX, USA) was used for immunoassays, which were performed as described previously [17, 18]. For antigen retrieval, the deparaffined sections were treated with 1 mM EDTA at pH 8.0 in an autoclave for five minutes. The sections were followed by incubation with 10% normal serum for 30 min, and incubated at 4°C for overnight with a primary antibody. The sections were washed with phosphate-buffered saline (PBS) and incubated with Alexa488-labeled anti-goat antibody (Thermo Fisher, Carlsbad, CA, USA) for 20 min at room temperature. After washing with PBS, the sections were mounted with crystal mount (Biomeda, Foster City, CA, USA) and observed under an LSM5 Pascal confocal microscope (Zeiss, Oberkochen, Germany).
Scoring method
We then quantified the glomerular expressions of nephrin according to a staining index [16]. Briefly, more than 20 glomeruli were chosen, and expression levels in each glomerulus were scored based on the extent of staining in the glomerulus, as follows: 0, none; 1, faint; 2, mild; 3, moderate; and 4, strong. The extent of nephrin level was expressed as the individual index (i.e. total score glomerular number examined) [18, 19].
Effects of candesartan and hydralazine administration
Male MSHRSP at five weeks of age were randomly divided into a control group (eight per group) and anti-hypertension drugs-treated group (six per group). 3.75-4.5 mg/kg/day of hydralazine hydrochrolide (30 mg/L, Wako Chemicals, Osaka, Japan) and 1.25-1.5 mg/kg/day of candesartan cilexetil (10 mg/L, Cayman Chemical Company, CO, USA) were administered in drinking water for 10 weeks from 6 weeks of age. Some MSHRSP died before 15 weeks of age due to apoplexy, and these rats were excluded.
Statistical analyses
The significance of differences was evaluated by the Student t-test. Values are expressed as the mean ± standard deviation, and P values < 0.05 were considered significant.
Results
Nephrin expressions in human kidney diseases
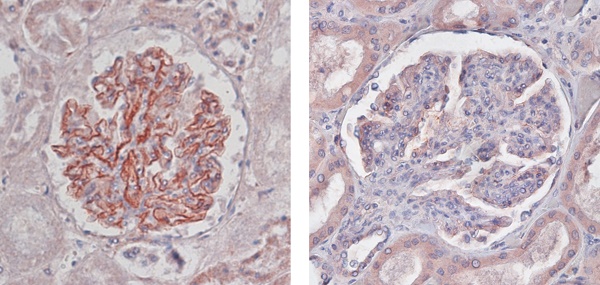
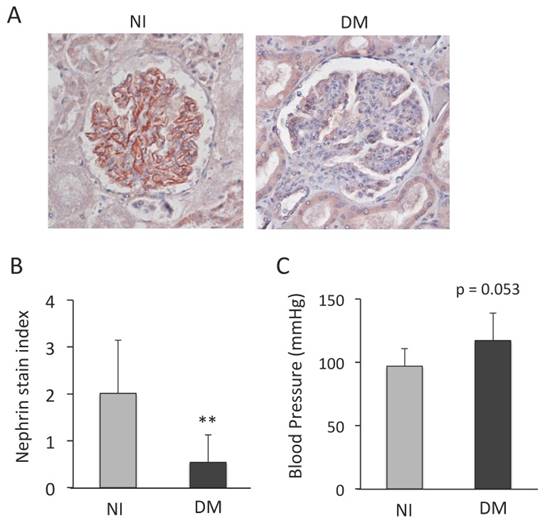
We used the specimens diagnosed with DN independent on this study and the control tissues of patients who had died of diseases without clinical or histological evidence of kidneys. In non-injured (NI) kidneys, we could detect the nephrin expression in many glomeruli (Fig. 1A). However, we found the low or no expression of nephrin in DN (Fig. 1A). The score of nephrin expression was significantly smaller in the specimens with DN than those with NI (Fig. 1B), and thus nephrin was reduced in human DN glomeruli. Actually, we investigated whether the specimens with DN had higher BP those with NI (Fig. 1C).
Nephrin expression in human glomeruli with diabetic nephropathy (DN). (A) Representative images of nephrin. Non-injured glomerulus (NI) and DN. (A; x400) (B) Quantification of nephrin expression in the glomeruli. Values are expressed as the mean ± SD. ** P < 0.01 for the comparison to the DN glomeruli with NIs. NI: n = 10, DN: n = 9. (C) Quantification of systolic BP. Values are expressed as the mean ± SD. NI: n = 8, DN: n = 6.
Effects of hydralazine on BP and kidney injury
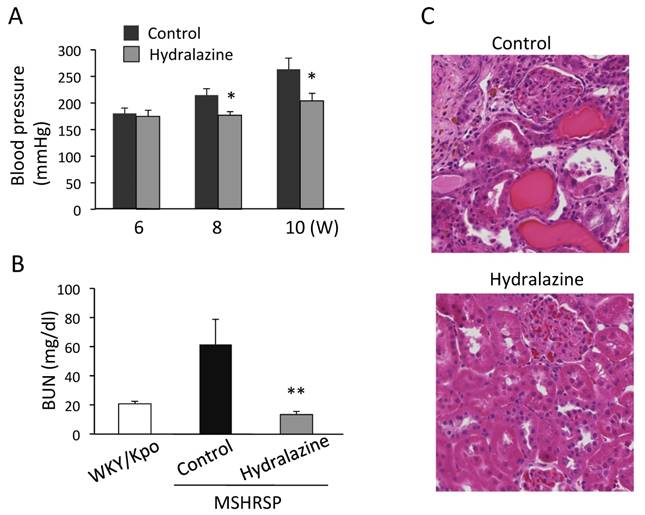
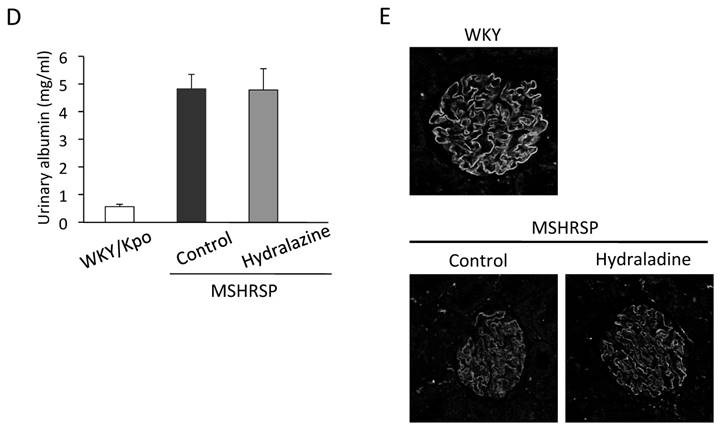
To investigate the molecular mechanisms of podocyte injuries in nephropathies, we subsequently examined the effects of a vasodilator, hydralazine, on podocyte injuries and proteinuria. We previously reported that the BP of MSHRSP was more than 180 mmHg at 6 weeks of age [11]. By treatment with hydralazine, the BP increase was prevented in the MSHRSP (Fig. 2A). The BUN value suggests that hydralazine treatment was effective to protect the renal functions (Fig. 2B). Also, the histological analyses show that hydralazine treatment prevents tubular injuries in MSHRSP (Fig. 2C). Therefore, hypertension is correlated with renal tubular cell injuries. Next, we investigated whether hydralazine suppressed the glomerular injuries in hypertensive nephropathy. As previously reported [11], control MSHRSP exhibited proteinuria as assessed by SDS-PAGE (Fig. 2D). Thus, we examined the effects of hydralazine on proteinuria at 15 weeks, but could not find significant differences between control- and hydralazine-treated MSHRSP (Fig. 2D). Moreover, hydralazine did not sufficiently prevent the decreased expression of nephrin by comparing with that in WKY (Fig. 2E). These results show that podocyte injuries do not depend on higher BP in MSHRSP.
Effects of hydralazine treatment on BP, proteinuria, and kidney injuries of MSHRSP. (A) BP of the control- and hydralazine-treated MSHRSP. (B) BUN in the hydralazine-treated MSHRSP at 15 weeks of age is compared with WKY and control MSHRSP. (C) Hematoxylin & Eosin (H&E) staining of control- and hydralazine-treated MSHRSP at 15 weeks of age. (x200) (D) Albumin concentration 24-hour urine. (E) Immunofluorescence assays of nephrin in the glomeruli of control- and hydralazine-treated MSHRSP compared with WKY. (x400). Values are expressed as the mean ± SD. * P < 0.05 and ** P < 0.01 for the comparison to the hydralazine-treated MSHRSP with controls. Control MSHRSP; n = 5, hydralazine-treated MSHRSP; n = 6.
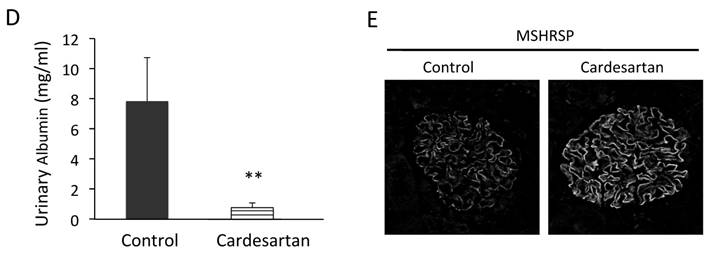
Effects of candesartan treatment on BP, proteinuria, and kidney injuries of MSHRSP. (A) BP of the control- and candesartan-treated MSHRSP. (B) BUN in the candesartan-treated MSHRSP at 15 weeks of age is compared with control. (C) H&E staining of control- and candesartan-treated MSHRSP at 15 weeks of age. (x200). (D) Albumin concentration in 24-hour urine. (E) Immunofluorescence assays of nephrin in the glomeruli of control- and candesartan-treated MSHRSP. (x400). Values are expressed as the mean ± SD. * P < 0.05 and ** P < 0.01 for the comparison to the candesartan-treated MSHRSP with controls. Control MSHRSP; n = 6, candesartan-treated MSHRSP; n = 6.
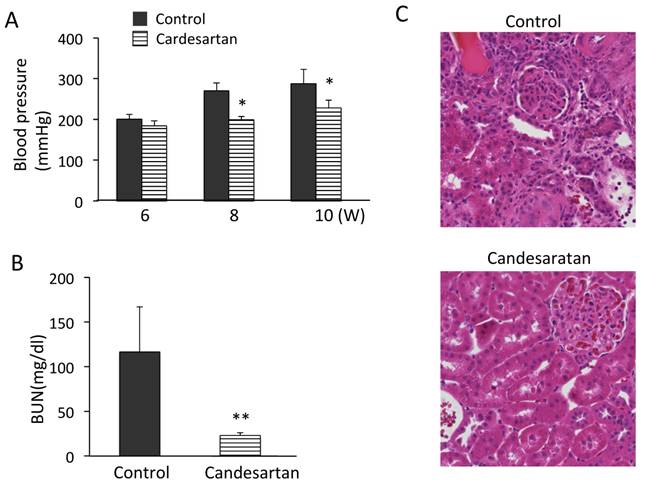
Effects of candesartan on BP and kidney injury
We further investigated the effects of an Ang receptor blocker (ARB), candesartan hydrochloride, on podocyte injuries. BP was significantly lower in the candesartan-treated group than in the control group (Fig. 3A). The renal function was protected in the candesartan-treated group based on measuring BUN (Fig. 3B). Histological analysis revealed the prevention of renal tubular cell injuries in MSHRSP (Fig. 3C). We next investigated whether candesartan inhibited the podocyte injuries in hypertensive nephropathy. Although the control MSHRSP exhibited proteinuria, treatment with candesartan resolved the proteinuria (Fig. 3D). Moreover, candesartan treatment was also found to maintain the nephrin expression in MSHRSP glomeruli (Fig. 3E). Therefore, excessive RAS system contributes the progression of both the tubular and podocyte injuries, and leads into nephrin loss and proteinuria. Therefore, ARB could play therapeutic effects in hypertensive nephropathy.
Discussion
Hypertension results in a complex of progressive histological changes in kidneys recognized as arteriosclerosis, cortical fibrosis, tubular atrophy and loss, and glomerulosclerosis that are pathological features referred to hypertensive nephropathy [5]. Of note, nephrin was decreased in human proteinuric nephropathy [20-22]. We in the present study confirmed that nephrin was reduced in the DN glomeruli. However, it is not well unknown as to the mechanisms of this nephrin loss. Welsh et al. previously reported that the nephrin expression was reduced in diabetes, and nephrinuria were actually detected in all diabetic patients with albuminuria [23]. Also, nephrinuria significantly correlated with systolic BP [23]. On the other hand, Toyoda et al. reported that the low expression of nephrin mRNA may be closely linked to development and/or progression of proteinuria in human DN [22]. Moreover, experimental and clinical observations suggest that podocyte damages, which is characterized by insufficiency of related proteins and/or reduction of podocytes, is involved in the occurrence and development of DN [1]. Under the basal condition and in acquired glomerular diseases, nephrin is required to maintain SD integrity and SD-mediated signaling to preserve glomerular function and podocyte viability in adult mice [24]. However, we could not observe the significant reduction of another podocyte protein, CD2AP in the glomeruli of DN (data not shown). Therefore, we thought it difficult to suppose the podocyte loss from the DN glomeruli.
Miceli et al. reported that mechanical stretch decreased nephrin expression in human podocytes [25]. Furthermore, human immortalized podocytes were subjected to repeated stretch-relaxation cycles by mechanical deformation with the use of a stress unit. Indeed, podocyte stretching induced both Ang II secretion and AT1 receptor overexpression, podocyte exposure to Ang II reduced nephrin expression, and the ARB, candesartan, completely abolished stretch-induced nephrin loss. Therefore, the increased expression of nephrin may contribute to the anti-proteinuric functions of RAS inhibitor. Actually, this theory is supported by in vivo experiments. Ang II infusion caused decreased nephrin expression [26, 27], and thus treatment with ARB caused increased glomerular nephrin expression and reduced albuminuria [28]. These effects were not found with an equally hypotensive dose of the calcium channel blockers, amlodipine and verapamil [29, 30]. Similarly, this increase was not significantly attenuated in the hydralazine-treated group, despite a similar reduction of the BP. Also, proteinuria in MSHRSP was completely suppressed in the candesartan group, confirming the effectiveness of RAS inhibition for the suppression of proteinuria in this model. Therefore, hypertension or mechanical stretch might be responsible for tubular injuries and dysfunctions, but may not be directly responsible for the nephrin down-regulation and proteinuria.
The hydralazine, which lowered BP to levels similar to those observed in candesartan-treated MSHRSP, was effective in reducing renal tubular injuries in MSHRSP. Therefore, we think that hypertension plays an essential role for the development of renal dysfunctions and tubular injuries in the MSHRSP kidney, while increase level of Ang II plays essential roles in the development of podocyte injuries and proteinuria. It is not clear as to the detailed molecular mechanisms. We think it is involved with endothelial damage. The extent of kidney damage due to hypertension is proportional to the degree of arterial pressure exposure of renal microvasculature [31]. As the collapse of capillary system, disappearance of whole vessels may result in reduction of tissue perfusion and consequent hypoxia. Therefore, in elevated BP, endothelial damages could be a main process toward tissue hypoxia and renal dysfunction with renal parenchymal injuries.
In the present study, we confirmed the nephrin was actually lost in the human DN glomerulus tuft. Furthermore, we examined the effects of hydralazine and candesartan in the proteinuria of MSHRSP rats, and found that hydralazine was effective for tubular injuries but not podocyte injuries, but candesartan had effects on both the tubular and podocyte injuries and, thus proteinuria suppression. Therefore, podocyte injuries were dependent on an excessive angiotensin system and not mechanical stress.
Acknowledgements
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 14454911 to TK) and a grant from the Osaka Kidney Foundation (OKF16-0006 to TK).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39-45
2. Haraldsson B, Nyström J. The glomerular endothelium: new insights on function and structure. Curr Opin Nephrol Hypertens. 2012;21:258-63
3. D'Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Current Opinion of Nephrology and Hypertension. 2008;17:271-81
4. Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189-95
5. Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Journal of Clinical and Experiment Nephrology. 2003;7:255-9
6. Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl). 1995;192:385-97
7. Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H. et al. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575-82
8. Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1-8
9. Takayanagi R, Inoguchi T, Ohnaka K. Clinical and experimental evidence for oxidative stress as an exacerbating factor of diabetes mellitus. J Clin Biochem Nutr. 2011;48:72-7
10. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H. et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-59
11. Kato T, Mizuguchi N, Ito A. Characteristics of podocyte injury in malignant hypertensive nephropathy of rats (MSHRSP/Kpo strain). Biomed Res. 2015;36:313-21
12. Berl T. Review: renal protection by inhibition of the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2009;10:1-8
13. Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266-70
14. Stojiljkovic L, Behnia R. Role of renin angiotensin system inhibitors in cardiovascular and renal protection: a lesson from clinical trials. Curr Pharm Des. 2007;13:1335-45
15. Kato T, Mizuguchi N, Ito A. Blood pressure, renal biochemical parameters and histopathology in an original rat model of essential hypertension (SHRSP/Kpo strain). Biomed Res. 2015;36:169-77
16. Kato T, Mizuno S, Nakamura T. Preservations of nephrin and synaptopodin by recombinant hepatocyte growth factor in podocytes for the attenuations of foot process injury and albuminuria in nephritic mice. Nephrology (Carlton). 2011;16:310-8
17. Kato T, Mizuno S, Kamimoto M. The decreases of nephrin and nuclear WT1 in podocytes may cause albuminuria during the experimental sepsis in mice. Biomed Res. 2010;31:363-9
18. Kato T, Mizuno S, Taketo MM, Kurosawa TM. The possible involvement of tensin2 in the expression and extension of nephrin by glomerular podocytes in mice. Biomed Res. 2008;29:279-87
19. Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-beta1 production by HGF in attenuation of murine diabetic nephropathy. Am J Physiol Renal Physiol. 2004;286:F134-43
20. Furness PN, Hall LL, Shaw JA, Pringle JH. Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant. 1999;14:1234-7
21. Gagliardini E, Benigni A, Tomasoni S, Abbate M, Kalluri R, Remuzzi G. Targeted downregulation of extracellular nephrin in human IgA nephropathy. Am J Nephrol. 2003;23:277-86
22. Toyoda M, Suzuki D, Umezono T, Uehara G, Maruyama M, Honma M. et al. Expression of human nephrin mRNA in diabetic nephropathy. Nephrol Dial Tranplant. 2004;19:380-5
23. Welsh GI, Saleem MA. Nephrin-signature molecule of the glomerular podocyte? J Pathol. 2010;220:328-37
24. Li X, Chuang PY, D'Agati VD, Dai Y, Yacoub R, Fu J. et al. Nephrin Preserves Podocyte Viability and Glomerular Structure and Function in Adult Kidneys. J Am Soc Nephrol. 2015;26:2361-77
25. Miceli I, Burt D, Tarabra E, Camussi G, Perin PC, Gruden G. Stretch reduces nephrin expression via an angiotensin II-AT(1)-dependent mechanism in human podocytes: effect of rosiglitazone. Am J Physiol Renal Physiol. 2010;298:F381-90
26. Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N. et al. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500-7
27. Yang Q, Ma Y, Liu Y, Liang W, Chen X, Ren Z. et al. Angiotensin II down-regulates nephrin-Akt signaling and induces podocyte injury: role of c-Abl. Mol Biol Cell. 2016;27:197-208
28. Davis BJ, Cao Z, de Gasparo M, Kawachi H, Cooper ME, Allen TJ. Disparate effects of angiotensin II antagonists and calcium channel blockers on albuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens. 2003;21:209-16
29. Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D. et al. Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte. J Hypertens. 2010;28:1034-43
30. Liang W, Chen C, Shi J, Ren Z, Hu F, van Goor H. et al. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant. 2011;26:789-99
31. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28:74-9
Author contact
![]() Corresponding author: Dr. Takashi KATO, Urologic Oncology Branch, Center for Cancer Research Building 10, Center Drive, MSC 1107, Bethesda, MD, 20892-1107, USA. E-mail: takashi0920kcom
Corresponding author: Dr. Takashi KATO, Urologic Oncology Branch, Center for Cancer Research Building 10, Center Drive, MSC 1107, Bethesda, MD, 20892-1107, USA. E-mail: takashi0920kcom