J Biomed 2017; 2:101-108. doi:10.7150/jbm.19770 This volume Cite
Research Paper
Anti-inflammatory Effect of JBP485 on Dextran Sulfate Sodium-induced Colitis in Mice
1. Department of Medical Pharmaceutics, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan;
2. Educational Center for Clinical Pharmacy, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan;
3. Department of Organic and Medicinal Chemistry, School of Pharmaceutical Sciences, Aichi Gakuin University, 1-100 Kusumoto-cho, Chikusa-ku, Nagoya 464-8650, Japan;
4. Endoscopy Department, Kindai University Nara Hospital, 1248-1, Otoda-cho, Ikoma 630-0293, Japan;
5. Department of Medical Biochemistry, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan.
Received 2017-2-22; Accepted 2017-5-24; Published 2017-6-21
Abstract
JBP485 (cyclo-trans-4-L-hydroxyprolyl-L-serine), a dipeptide first isolated from Laennec that is a proprietary product made from the hydrolysate of human placenta, has been reported to have several biological properties, such as anti-hepatotoxic, anti-inflammatory and anti-apoptotic activities. However, the effect of JBP485 on inflammatory bowel disease has not yet been reported. Here we investigated the effects of simultaneous and subsequent administration of JBP485 on dextran sulfate sodium (DSS)-induced colitis in mice. In both experimental protocols, DSS-induced colitis was established by giving mice drinking water containing 2% (w/v) DSS for 5 days. JBP485 (25 mg/kg) was orally administered once a day during and after DSS treatment for 5 days in the simultaneous administration experiment while after DSS treatment for 5 days in the subsequent administration experiment. In the simultaneous administration experiment, JBP485 did not improve disease progression, such as weight loss, diarrhea and visible fecal blood, as well as shortening of the colon, but reduced the DSS-induced injury and up-regulation of tumor necrosis factor-α, interleukin-1β, cyclooxygenase-2, monocyte chemotactic protein 1 and C-X-C motif chemokine ligand 1 mRNAs in the colon. In the subsequent administration experiment, however, JBP485 did not show any effects on DSS-induced colitis. These results demonstrated that simultaneous administration but not subsequent administration of JBP485 had anti-inflammatory effects on DSS-induced colitis in mice.
Keywords: dextran sulfate sodium, gene expression, inflammation, inflammatory bowel disease.
Introduction
Incidence of inflammatory bowel disease (IBD), chronic inflammatory conditions of the gastrointestinal tract, represented by ulcerative colitis (UC) and Crohn's disease (CD), is currently increasing worldwide [1]. IBD is characterized by recurrent and long-lasting episodes of diarrhea, abdominal pain and fecal blood. The pathogenesis of IBD remains largely unknown, but it has been reported that a number of factors, such as genetic predispositions, intestinal microenvironment and immunological factors, are critically implicated [2-5]. In UC and CD, genetic and environmental factors likely trigger abnormal immune responses and excessive inflammatory reactions. Anti-inflammatory drugs, immunosuppressants and antibiotics are mainly used for the treatment for IBD, but many problems exist in such current therapies, including major adverse events and poor therapeutic responses. Therefore, the development of novel and safe therapies is a prime concern.
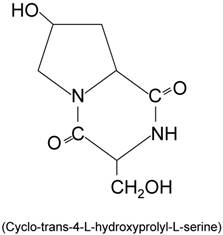
JBP485 (cyclo-trans-4-L-hydroxyprolyl-L-serine) (Fig. 1) is a dipeptide first isolated from Laennec [6]. Laennec is a proprietary product made from the hydrolysate of human placenta [7]. JBP485 has been reported to have several biological properties. In concanavalin A (Con A)-induced liver injury mouse model, administration of JBP485 reduced hepatic damage, such as increases in tumor necrosis factor (TNF)-α and intercellular adhesion molecule-1 expression and apoptosis in the liver [7]. Also, administration of JBP485 to rats improved gentamicin-induced acute renal failure [8]. However, the effect of JBP485 on IBD has not yet been reported.
Chemical structure of JBP485.
The purpose of this study was to investigate the effect of JBP485 on dextran sulfate sodium (DSS)-induced colitis in mice. We employed two protocols: (1) simultaneous and (2) subsequent administration of JBP485. In this study, we used salazosulfapyridine (SASP) as a control drug and attempted to compare the therapeutic effects of JBP with those of SASP on DSS-induced colitis in mice because SASP is clinically used for the treatment of IBD in humans.
Materials and Methods
Materials
JBP485 was chemically synthesized at Laboratory of Organic and Medicinal Chemistry, Aichi Gakuin University (Nagoya, Japan). SASP and 0.3% methylcellulose were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Saline were purchased from Otsuka, Pharmaceutical Co., Ltd. (Tokyo, Japan).
JBP485 and SASP preparation
JBP485 powder was dissolved in saline and stored at -80°C until used. SASP was suspended in 0.3% methylcellulose.
Animals
Male C57BL/6 mice (6 weeks old, n = 41) were purchased from CLEA Japan (Shizuoka, Japan). The mice were maintained under an artificial 12-h dark-light cycle (lights on at 8:30 AM) at a constant temperature of 24 ± 1°C and 55% humidity under specific-pathogen-free conditions. They were provided with the laboratory chow CE-2 (CLEA) and water ad libitum. All experimental procedures described were approved by the Animal Care Committee of Kobe Pharmaceutical University.
DSS-induced colitis model
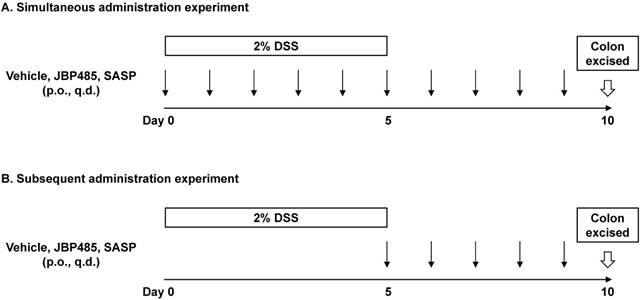
Study protocol was shown (Fig. 2). DSS-induced colitis model was established as described previously [9]. In brief, mice were provided with 2% (weight/volume) DSS (molecular weight, 36,000-50,000 Da; MP Biomedicals, Solon, OH, USA) in their drinking water for 5 days. Control mice received water without DSS. On day 5, DSS administration was ceased. On day 10 (5 days after cessation of DSS administration), mice were euthanized under anesthesia (isoflurane inhalation) and then the proximal (2 cm distal to the cecum) and the distal colon (2 cm proximal to the anus) was excised and used for real-time PCR analysis. The severe inflammation was observed in the distal colon but not in the proximal colon (Supplemental Fig. 1) [10]. Therefore, we used the distal colon for the subsequent histological and real-time PCR analyses to elucidate the effect of JBP485 on DSS-induced colitis in mice. In the simultaneous administration experiment (Fig. 2A), 0.3% (wt/wt) methylcellulose (vehicle, Veh), JBP485 (25 mg/kg), or SASP (250 mg/kg) was orally administered to DSS-induced colitis model a day during and after DSS treatment for 5 days. In the subsequent administration experiment (Fig. 2B), they were orally administered to DSS-induced colitis model a day only after DSS treatment for 5 days. Mice were weighed and monitored for the appearance of diarrhea and blood in the stool throughout the experimental period.
Study protocol. (A, B) Two experiments were conducted to examine the protective and curative effects of JBP485 on DSS-induced colitis in mice. (A) Simultaneous administration experiment. (B) Subsequent administration experiment. p.o.: per os., q.d.: quaque die.
Assessment of a disease activity index (DAI)
Disease activity was assessed as described previously [11, 12]. In brief, body weight, stool consistency, and blood in the stool were monitored daily using the criteria described in Table 1. Disease activity index (DAI) was shown the combined score of weight loss, stool consistency and bleeding.
DAI score
| Score | Weight loss | Stool consistency | Blood in stool |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1-5% | ||
| 2 | 5-10% | loose stools | Slight bleeding |
| 3 | 10-15% | ||
| 4 | >15% | Watery diarrhea | Gross bleeding |
Histopathological analysis
The distal colon was removed and fixed with 10% neutral buffered formalin and embedded in paraffin. Paraffin-embedded tissue sections were cut 4 μm thick and stained with haematoxylin and eosin (H-E) to address the degree of inflammation. Histology was scored as follows: epithelium damage, 0 = normal morphology; 1 = loss of globlet cells; 2 = loss of globlet cells in large areas; 3 = loss of crypts; 4 = loss of crypts in large areas; and infiltration, 0 = no infiltrate; 1 = infiltrate around the crypt basis; 2 = infiltrate reaching the lamina muscularis mucosae; 3 = extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant edema; 4 = infiltration of the lamina submucosa [12].
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from the distal colon using TRIzol™ Reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instructions. RNA concentration was determined using a ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). One micrograms of total RNA were used cDNA synthesis. A reverse transcription was performed as described in ReverTra Ace® qPCR RT kit (Toyobo, Osaka, Japan) protocol. cDNA was amplified with Thunderbird® SYBR® Green PCR Master Mix (Toyobo). PCR conditions were 50 cycles of 15 s at 95°C for denaturation and 60 s at 60°C for annealing and extension. Primer sequences are shown in Table 2. Relative expression was calculated using the delta-delta Ct method with β-actin used as a reference gene [13].
Primer sequences
| Gene | NM_ID | Product size (bp) | Sequence (5'→3') | |
|---|---|---|---|---|
| Tnfa | NM_013693.2 | 103 | Fw | CCACCACGCTCTTCTGTCTAC |
| Rv | AGGGTCTGGGCCATAGAACT | |||
| Il1b | NM_008361.3 | 152 | Fw | CAACCAACAAGTGATATTCTCCATG |
| Rv | GATCCACACTCTCCAGCTGCA | |||
| Il6 | NM_031168.1 | 102 | Fw | CTGGAGTCACAGAAGGAGTGG |
| Rv | GGTTTGCCGAGTAGATCTCAA | |||
| Nos2 | NM_010927.3 | 95 | Fw | CAGCTGGGCTGTACAAACCTT |
| Rv | CATTGGAAGTGAAGCGTTTCG | |||
| Ptgs2 | NM_011198.3 | 129 | Fw | GGTGCCTGGTCTGATGATGTATG |
| Rv | ATGAGTATGAGTCTGCTGGTTTGG | |||
| Ccl2 | NM_011333.3 | 95 | Fw | GCATCCACGTGTTGGCTCA |
| Rv | CTCCAGCCTACTCATTGGGATCA | |||
| Cxcl1 | NM_008176 | 111 | Fw | ACCGAAGTCATAGCCACACTC |
| Rv | CCGTTACTTGGGGACACCTT | |||
| Actb | NM_009898.2 | 138 | Fw | AGAGGGAAATCGTGCGTGAC |
| Rv | CAATAGTGATGACCTGGCCGT |
Statistical analyses
Data are the mean ± standard error (SEM) of independent determinations for each experiment. The comparisons of more than two groups were evaluated by one-way analysis of variance followed by the Tukey's test with SPSS v22.0. A value of P<0.05 was considered significant.
Results
Effect of simultaneous administration of JBP485 on disease activity index and colon length
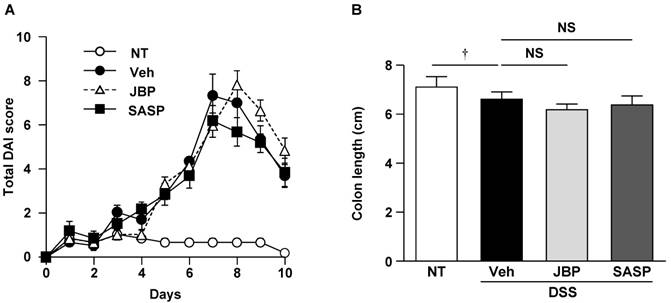
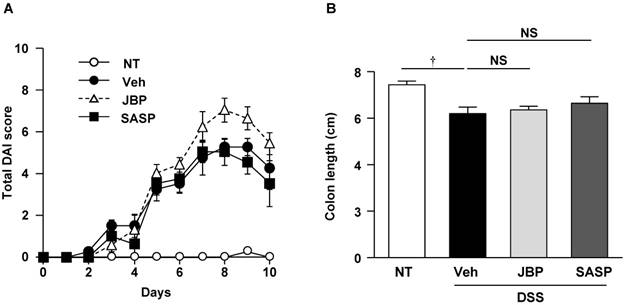
We investigated whether simultaneous administration of JBP485 had a protective effect on DSS-induced colitis in mice. This model is characterized by weight loss, diarrhea or loose feces, and visible fecal blood. The protective effect of JBP485 on DSS-induced colitis was assessed using a DAI. Compared with the untreated (NT) group, DAI scores were significantly increased in the DSS-induced colitis (DSS+Veh) group (Fig. 3A). JBP485 tended to decrease the DAI score until day 4, but did not show a statistically significant difference. Administration of JBP485 alone to control mice did not affect body weight or stool throughout the experimental period (data not shown). SASP did not show any protective effect, either. Consistent with these results, JBP485 and SASP did not prevent the shortening of the colon (Fig. 3B). These results indicate that JBP485 did not show a protective effect on DSS-induced colitis.
Histopathological analysis of the effect of JBP485 on DSS-induced colitis
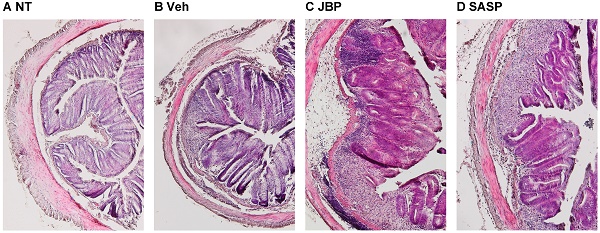
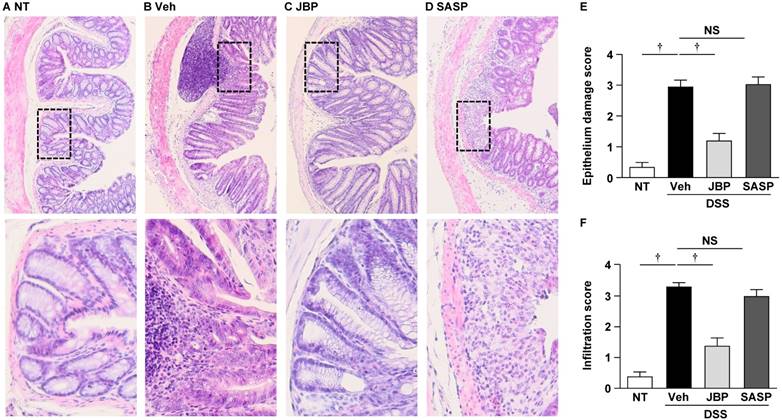
To investigate the effect of JBP485 on DSS-induced colitis in more detail, we performed histopathological analysis using H-E staining. Compared with the NT group, the DSS+Veh group showed severe damage in the intestinal epithelium, associated with pronounced decrease in number of crypts and infiltration of inflammatory cells (Fig. 4, A and B). However, JBP485 reduced the DSS-induced pathological changes, particularly inflammatory cell infiltration (Fig. 4C). SASP also displayed modest decrease (Fig. 4D). The histopathological injury scores were significantly decreased in the JBP485-treated (DSS+JBP485) and SASP-treated (DSS+SASP) groups compared with the DSS+Veh group (Fig. 4E and F). These results indicate that the simultaneous administration of JBP485 exhibited a protective effect on the DSS-induced pathological changes.
Effect of JBP485 on mRNA expression of pro-inflammatory molecules in the colon
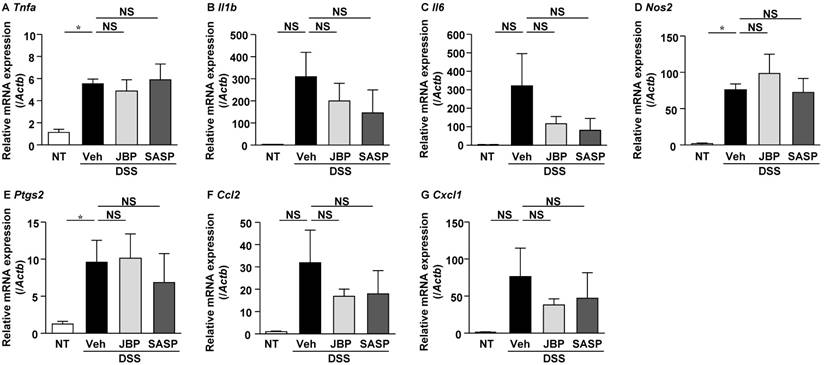
We then analyzed the mRNA expression levels of pro-inflammatory cytokines and chemokines by real-time PCR. Compared with the NT group, the mRNA expression levels of TNF-α, interleukin (IL)-1β, IL-6, monocyte chemotactic protein 1 (MCP-1) and C-X-C motif chemokine ligand 1 (CXCL1) were significantly increased in the DSS+Veh group (Fig. 5, A-C, F and G). JBP485 significantly decreased the mRNA expression levels of TNF-α, IL-1β, MCP-1 and CXCL1 but not of IL-6. SASP did not decrease the mRNA expression levels of TNF-α, IL-1β and IL-6, or rather increased the TNF-α mRNA expression level. Administration of JBP485 alone to control mice did not affect the mRNA expression levels of TNF-α, IL-1β and IL-6 (Supplemental Fig. 2).
Effect of simultaneous administration of JBP485 on disease activity index and colon length. (A) Disease activity index. Mice were treated with 2% DSS plus vehicle (Veh), 2% DSS plus JBP485 (JBP), or 2% DSS plus SASP (SASP), or untreated (NT) and total disease activity index was assessed daily. (B) Effect of JBP485 on the shortening of the colon. The entire colon length of mice treated with 2% DSS plus vehicle (Veh), 2% DSS plus JBP485 (JBP), or 2% DSS plus SASP (SASP), or untreated (NT) for 5 days was measured. n = 6, each group.
Histopathological analysis of the effect of simultaneous administration of JBP485 on DSS-induced colitis. (A-D) Representative images of histological analysis with H-E staining. Untreated (NT, A), 2% DSS plus vehicle (Veh, B), 2% DSS plus JBP485 (JBP, C), 2% DSS plus SASP (SASP, D). Lower images represent enlarged images of the area enclosed by the dashed lines. (E) Epithelium damage score. (F) Infiltration score. n = 6, each group.
Effect of simultaneous administration of JBP485 on mRNA expression of pro-inflammatory molecules in the colon. mRNA expression levels of TNF-α (Tnfa, A), IL-6 (Il6, B), IL-1β (Il1b, C), iNOS (Nos2, D), COX-2 (Ptgs2, E), MCP-1 (Ccl2, F) and CXCL1 (Cxcl1, G) in the colon were analyzed using real-time PCR. n = 6, each group. *P<0.05; †P<0.01; NS: not significant.
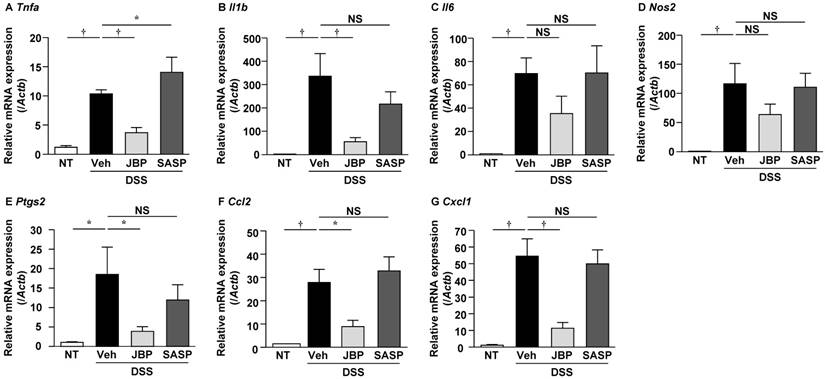
We also analyzed the mRNA expression levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 and found that both were significantly increased in the DSS+Veh group compared with the NT group (Fig. 5, D and E). JBP485 significantly decreased the mRNA expression levels of COX-2, but not of iNOS. SASP did not influence these mRNA expression levels.
Effect of subsequent administration of JBP485 on DSS-induced colitis
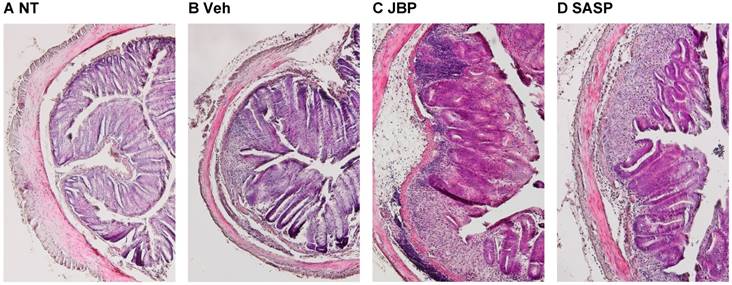
In the following experiments, we investigated whether JBP485 had a curative effect on DSS-induced colitis in mice. After DSS treatment, JBP485 was subsequently administered to mice for 5 days. The subsequent administration of JBP485 did not improve disease activity (Fig. 6A), the shortening of the colon (Fig. 6B) and the DSS-induced pathological changes (Fig. 7). JBP485 did not decrease the mRNA expression levels of pro-inflammatory cytokines, chemokines, COX-2 and iNOS (Fig. 8). These results indicate that the subsequent administration of JBP485 did not show a curative effect on DSS-induced colitis in mice.
Discussion
Here we showed that in the DSS-induced mouse colitis model the simultaneous administration of JBP485 reduced the inflammatory responses in the colon, although this treatment did not improve disease progression significantly. JBP485 is a dipeptide derivative from human placenta hydrolysates [7]. Orally administered JBP485 is suggested to be almost completely absorbed from gastrointestinal tract, and hardly to be removed by hepatic first-pass [6]. JBP485 is absorbed through the gastrointestinal tract by the di/tripeptide transporter PepT1 [14]. PepT1 is expressed highly in epithelial cells of the small intestine, but poorly or not at all in the colon [15]. However, it has been reported that PepT1 expression in the colon is up-regulated in chronic inflamed states including IBD [15, 16]. Thus, it is assumed that orally administered JBP485 can be absorbed well in the small intestine and presumably in the colon. It remains unclear whether absorbed JBP485 influences the colonic epithelial cells directly or indirectly through absorption in the small intestine.
Effect of subsequent administration of JBP485 on disease activity index and colon length. (A) Disease activity index. Mice were treated with 2% DSS plus vehicle (Veh), 2% DSS plus JBP485 (JBP), or 2% DSS plus SASP (SASP), or untreated (NT) and total disease activity index was assessed daily. (B) Effect of JBP485 on the shortening of the colon. The entire colon length of mice treated with 2% DSS plus vehicle (Veh), 2% DSS plus JBP485 (JBP), or 2% DSS plus SASP (SASP), or untreated (NT) for 5 days was measured. n = 4, NT, Veh, and SASP groups; n = 5, JBP group.
Histopathological analysis of the effect of subsequent administration of JBP485 on DSS-induced colitis. Representative images of histological analysis with H-E staining. Untreated (NT, A), 2% DSS plus vehicle (Veh, B), 2% DSS plus JBP485 (JBP, C), 2% DSS plus SASP (SASP, D).
Effect of subsequent administration of JBP485 on mRNA expression of pro-inflammatory molecules in the colon. mRNA expression levels of TNF-α (Tnfa, A), IL-6 (Il6, B), IL-1β (Il1b, C), iNOS (Nos2, D), COX-2 (Ptgs2, E) and MCP-1 (Ccl2, F) and CXCL1 (Cxcl1, G) in the colon were analyzed using real-time PCR. n = 4, NT, Veh, and SASP groups; n = 5, JBP group. *P<0.05; NS: not significant.
It has been reported that JBP485 has tissue protective effects against the Con A-induced hepatotoxicity and the gentamicin-induced nephrotoxicity, and that these favorable actions of JBP485 likely result from anti-oxidative and anti-apoptotic activities of this peptide [7, 8]. In the present study JBP485 inhibited the up-regulation of TNF-α, IL-1β, MCP-1, CXCL1 and COX-2 mRNAs in the DSS-induced inflamed colon. Furthermore, JBP485 tended to decrease IL-6 and iNOS mRNAs, although the reduction of IL-6 or iNOS mRNA expression by JBP485 did not reach statistical significance. The differential degree of inhibitory action of JBP485 might be due to the differential time course of changes in these mRNA levels. In Con A-induced liver damage model, JBP485 inhibited the production and secretion of TNF-α, which is consistent with our results [7]. To reveal the molecular mechanism underlying the anti-inflammatory action of JBP485 against DSS-induced colitis, we assessed the effect of JBP485 on lipopolysaccharide-induced TNF-α mRNA expression in cultured colon epithelial cell line Caco-2 cells, but we could not observe anti-inflammatory effect in vitro (data not shown). Therefore, the direct action on epithelial cells might not play a major role in the anti-inflammatory action of JBP485 in mice. On the other hand, SASP, which is widely used to treat IBD, did not decrease TNF-α, IL-1β, IL-6, MCP-1, CXCL1, iNOS and COX-2 mRNA expression in DSS-induced inflamed colon. This is consistent with a previous report showing that SASP did not improve DSS-induced colitis in mice [10].
The mechanism of the DSS-induced colitis in mice and rats remains unclear, but it is suggested that DSS induces damage of the mucosal epithelial barrier, allowing the dissemination of pro-inflammatory intestinal contents, such as intestinal commensal bacteria and their products, into the colonic wall [9]. Pro-inflammatory intestinal contents cause the infiltration of inflammatory cells and increase pro-inflammatory cytokines, further enhancing penetration and dissemination of bacteria and their products. In addition to such a mechanism, innate immunity also plays a role in humans. Interestingly, JBP485 promoted the expression and secretion of mucin in the conjunctival epithelium [17]. Mucin plays an important role to preserve gastrointestinal mucosal barrier against bacterial penetration, and therefore it could be possible that JBP485 protects the DSS-induced damage of the mucosal epithelial barrier by promoting the expression and secretion of mucin. Thereafter, we examined whether mucin 2 mRNA expression could be up-regulated after administration of JBP485 for 5 days, but observed that it was not changed (98.6 ± 0.56 % of the control mice, n = 4). Consistent with this result, in the present study, administration of JBP485 did not improve significantly disease activity such as diarrhea and visible fecal blood. These results imply that JBP485 did not have a significant protective effect on DSS-induced mucosal barrier damage, but exhibited only anti-inflammatory action as shown with the reduction in inflammatory cell infiltration and in pro-inflammatory cytokine/chemokine induction.
Surprisingly, the mechanism of how DSS penetrates the epithelial cells is still unknown as DSS could be absorbed through passive or active uptake via a specific receptor or through cell penetration after complexation with another molecular form. We showed here that the subsequent administration of JBP485 did not show a curative effect on DSS-induced colitis. Accordingly, the possibility that JBP485 reduces epithelial penetration of DSS could not be excluded.
What are clinical implications in this study? TNF-α may have a central role in immune-mediated diseases, such as rheumatoid arthritis and IBD, because inhibition of TNF-α by means of neutralizing antibodies or soluble decoy receptors has been proven for its favorable effect on these diseases. Because JBP485 markedly prevented TNF-α in the DSS-induced inflamed colon, the present study provides the possibility that this peptide emerges as a promising agent for IBD. However, unfortunately because the favorable effect of JBP485 on IBD requires further clarification, it might be used as an adjunctive therapy together with the primary treatment with anti-inflammatory and immunosuppressant drugs.
In conclusion, in the simultaneous administration experiment, JBP485 did not improve disease activity significantly, but did reduce the DSS-induced injury and up-regulation of TNF-α, IL-1β, MCP-1, CXCL1 and COX-2 mRNAs in the colon. In the subsequent administration experiment, however, JBP485 did not show any effects on DSS-induced colitis. These results provide new insights into the anti-inflammatory action of simultaneous administration of JBP485 in DSS-induced colitis.
Supplementary Material
Supplementary figures.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Molodecky NA, Soon IS, Rabi DM. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42 quiz e30
2. Di Sabatino A, Biancheri P, Rovedatti L. et al. Recent advances in understanding ulcerative colitis. Intern Emerg Med. 2012;7:103-11
3. Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne). 2014;1:3
4. Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap Adv Gastroenterol. 2015;8:66-82
5. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205-17
6. Liu KX, Kato Y, Kaku TI. et al. Hydroxyprolylserine derivatives JBP923 and JBP485 exhibit the antihepatitis activities after gastrointestinal absorption in rats. J Pharmacol Exp Ther. 2000;294:510-5
7. Yang T, Wu J, Wang C. et al. Protective effect of JBP485 on concanavalin A-induced liver injury in mice. J Pharm Pharmacol. 2009;61:767-74
8. Guo X, Meng Q, Liu Q. et al. JBP485 improves gentamicin-induced acute renal failure by regulating the expression and function of Oat1 and Oat3 in rats. Toxicol Appl Pharmacol. 2013;271:285-95
9. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25
10. Liu J, Xiao HT, Wang HS. et al. Halofuginone reduces the inflammatory responses of DSS-induced colitis through metabolic reprogramming. Mol Biosyst. 2016;12:2296-303
11. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-49
12. Yoshihara K, Yajima T, Kubo C, Yoshikai Y. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut. 2006;55:334-41
13. Horibe S, Tanahashi T, Kawauchi S. et al. Preventative Effects of Sodium Alginate on Indomethacin-induced Small-intestinal Injury in Mice. Int J Med Sci. 2016;13:653-63
14. Cang J, Zhang J, Wang C. et al. Pharmacokinetics and mechanism of intestinal absorption of JBP485 in rats. Drug Metab Pharmacokinet. 2010;25:500-7
15. Merlin D, Si-Tahar M, Sitaraman SV. et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666-79
16. Wojtal KA, Eloranta JJ, Hruz P. et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871-7
17. Nakamura T, Hata Y, Nagata M. et al. JBP485 promotes tear and mucin secretion in ocular surface epithelia. Sci Rep. 2015:5
Author contact
![]() Corresponding author: Yoshiyuki Rikitake, MD, PhD, Department of Medical Pharmaceutics, Kobe Pharmaceutical University 4-19-1, Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan. Tel: +81-78-441-7578, Fax: +81-78-441-7578, E-mail: rikitakeac.jp
Corresponding author: Yoshiyuki Rikitake, MD, PhD, Department of Medical Pharmaceutics, Kobe Pharmaceutical University 4-19-1, Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan. Tel: +81-78-441-7578, Fax: +81-78-441-7578, E-mail: rikitakeac.jp