J Biomed 2018; 3:19-25. doi:10.7150/jbm.23068 This volume Cite
Research Paper
Lipoprotein-Associated Phospholipase A2 Activity Level may be complementary to Cardiactroponin I as a Biomarker for Acute Myocardial Infarction in Chinese Patients with Chest Pain
Department of Cardiology, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine. Shanghai, P.R. China
Received 2017-11-25; Accepted 2017-12-2; Published 2018-2-16
Abstract
Background: Lipoprotein-associated phospholipase A2 (Lp-PLA2) is associated with increased atherosclerotic cardiovascular disease (ASCVD) risk, which relatively unique in its high specificity for vascular inflammation. So we study if Lp-PLA2 is independent of or complementary to cardiac troponin I (cTn I) as a risk predictor of acute myocardial infarction (AMI).
Methods: Across-sectional analysis on 125 patients admitted to the cardiovascular department for acute chest pain, including age, gender, family history, smoking status, hypertension, diabetes mellitus, kidney function, cTn I, blood lipid and Lp-PLA2 activity. Univariate and multivariate logistic regression analyses and the Cox proportional hazards model were performed to identify the risk factors for ASCVD.
Results: Lp-PLA2 activity was higher in AMI than stable / unstable angina pectoris (UA/SA). (173±59U/L vs 144±46U/L, P< 0.01).The area under the ROC curve (AUC) of Lp-PLA2 activity used to predict AMI was 0.649 (95% CI: 0.546 - 0.752, P=0.007). The Lp-PLA2 cut-off value was 157 IU/L, and the sensitivity and specificity were 60% and 71%, respectively. Lp-PLA2 level was associated with the prevalence of AMI with an odds ratio (OR) of 2.723 [95% confidence interval (CI): 1.145‒6.473, p=0.023] in multivariate analysis.
Conclusion: Lp-PLA2activity was independent of cTnI and may be complementary to cTnI as an AMI risk marker.
Keywords: Lipoprotein-associated phospholipase A2, atherosclerotic coronary artery disease, risk predictor, blood lipid
Introduction
Acute myocardial infarction (AMI) is one of the most common diagnoses in hospitalized patients in industrialized countries. Mortality is approximately fourfold higher in elderly patients (over age 75) as compared with younger patients (Krumholz et al. 2001). Delay in diagnosis and management results in a high mortality, and prompt early diagnosis rates and interventions may be life-saving. Early identification of myocardial infarction in patients with atherosclerotic cardiovascular disease (ASCVD) has a great clinical significance.
The marker of myocardial injury is the most frequently used diagnostic method in heart disease patients. Cardiac troponinis a kind of contractive and adjustive protein exists in the thin myocardial fibre, which subunits will diffuse into venous blood when the myocardial-cell injured (Zheng et al. 2011, Acevedo et al. 2015, Donato et al. 2015). As a highly sensitive and specific indicator of myocardial injury, Cardiac troponin I (cTn I) is a well-known marker for the diagnosis of acute myocardial infarction (AMI). However, cTnI appears to be relatively low in its specificity for myocardial infarction. Increased cTnI is also seen in other cardiovascular diseases and non-cardiovascular diseases, such as heart failure, myocarditis, pulmonary embolism, renal insufficiency and so on (Cardinaels et al. 2016). In order to improve the early identification and accuracy of AMI, it is need for new risk predictor to able identify AMI or be complementary to cTnI.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is associated with increased cardiovascular diseases risk, which relatively unique in its high specificity for vascular inflammation (Chung et al. 2014, Maiolino et al. 2015, Wei et al. 2017). Lp-PLA2, produced by macrophages and lymphocytes, binds to low density lipoprotein, which can hydrolyze oxidized phospholipids on low density lipoprotein, and produce free fatty acids and lyolecithin (Wu et al. 2016). Lp-PLA2 promotes the occurrence and development of inflammation and atherosclerosis. Recent studies have demonstrated the level of Lp-PLA2 was not only related to the pathogenesis of atherosclerosis, but also to plaque stability and to the severity of coronary artery stenosis (Caslake et al. 2010). The level of Lp-PLA2 in unstable plaque or ruptured plaque was higher than that in stable plaque. Comparison of Lp-PLA2 with traditional inflammatory markers and myocardial injury marker, the diagnostic value of early diagnosis was higher than that of traditional inflammatory markers (Caslake et al. 2010). Based on this evidence, we proposed the use of Lp-PLA2 as a cardiovascular risk marker. The association between higher Lp-PLA2 and CVD risk has been previously reported in a multi-ethnic population (Garg et al. 2015). However, Higher Lp-PLA2 mass and activity were both associated with increased incidence of CVD and CHD in Blacks, Hispanics, and Whites but not in Chinese. So, we evaluated associations of Lp-PLA2 activity with atherosclerotic cardiovascular disease in Chinese individuals. In this study we investigated Lp-PLA2 level and compared with cTnI in ASCVD, and analyzed if Lp-PLA2 could be an independent marker for the risk of AMI.
Materials and Methods
Study population
In this study, across-sectional analysis on 125 consecutive patients with chest pain admitted to the cardiovascular department from January 2016 to July 2016. Coronary angiography was performed to figure out the degree of coronary artery stenosis. The study protocol was approved by the Shanghai Ninth People's Hospital Ethics Committee and Research Board. Written informed consent was obtained from all study participants.
Clinical presentations in terms of stable angina pectoris (SA) or unstable angina pectoris (UA) and acute myocardial infarction (AMI) were diagnosed according to clinical manifestation, electrocardiography findings and cardiac biomarkers by two experts of cardiology. Demographic characteristics including age, gender, family history of ASCVD, smoking status, hypertension and diabetes mellitus were recorded by questionnaire and were double-checked by two experts of cardiology. The serum total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), Kidney function, cardiac troponin, creatinine kinase and fasting blood glucose were measured using standard laboratory techniques, immediately after admission. All assays were conducted by a laboratory scientist according to the manufacturer's specification.
Analysis of Lp-PLA2 activity
The venous blood samples were collected at the time of catheterization prior to angiography. For plasma samples blood was collected in EDTA vials and separation of plasma was done by centrifugation at 3000 rpm at 4°C for 10 min. The plasma samples were stored at -80°C and analyzed later for activity of Lp-PLA2. Lp-PLA2 activity was measured by using a modification of spectrophotometric assay (Cayman Chemicals MI, USA) which used thiol derivative of platelet activating factor (PAF) as a substrate (Parveen et al. 2015). The generation rate of p-nitro phenol was determined at 405 nm, 25°C, with the use of a continuously recording spectrophotometer (Beckman DU-68) (Garg et al. 2015).The intra-and inter assay coefficients of variance for the Lp-PLA2activity assay used were both < 3.5%, as determined from 30 replicates performed on 15 different days during the course of the sample analyses.
Statistical analysis
Statistical tests were performed using SPSS (Statistical Package for the Social Sciences) version 17 software (SPSS Inc., Chicago, Illinois, USA). Data are presented as a percentage or mean value ± standard error. The frequencies of categorical variables are represented as counts (percentages), and the continuous variables are represented as either the mean ± standard deviation or the median with interquartile range. Multiple group comparisons were compared via Student's-tests (normally distributed) or nonparametric tests (non-normally distributed) for continuous variables and χ2 tests for categorical variables. The Spearman correlation was performed with each biomarker, adjusted for age and gender. Univariate and multivariate logistic regression analyses were performed to identify the risk factors for ASCVD. Both the logistic regression model and the Cox proportional hazards model were used. According to the size of the area under the ROC curve (AUC), receiver operating characteristic (ROC) analyses were used to summarize the diagnostic power of Lp-PLA2 for AMI patients. Optimal cut-offs were derived from the ROC curves by maximizing the sum of sensitivity and specificity. Two sided p values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
This study included 125 subjects (36% women) with a mean age of 69.7 ± 11.2 years. The baseline characteristics and selected laboratory values of the study patients are presented in Table 1. There were no significant differences in age, history of hypertension, smoking, diabetes mellitus, heart failure, lipid drugs medication (%), HDL cholesterol and triglycerides levels between AMI and SA/UA group. The AMI patients had significantly higher total cholesterol, LDL-C, serum creatinine compared with the SA/UA patients.
CK-MB, cTnI and Lp-PLA2 activity level
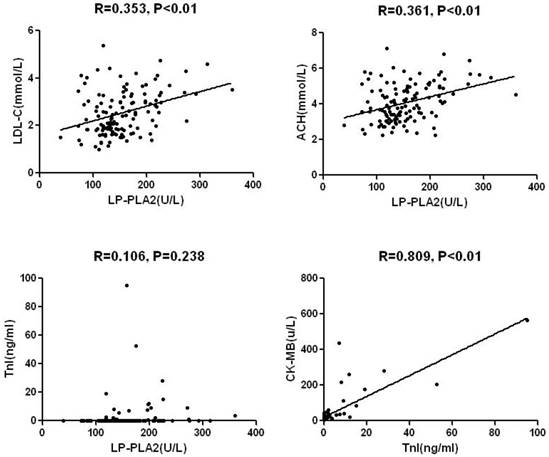
Mean level of Lp-PLA2 activity was 154±54U/L in the total sample with significant differences between AMI and SA/UA: 173±59U/L and 144±46U/L (P< 0.01), respectively (Table 1). Lp-PLA2 was significantly and directly correlated with LDL-C, non-HDL-C, and plasma creatinine (Fig.2 and Table 2). No significant correlations were found between Lp-PLA2 and age, blood glucose, CK-MB, cTnI, and HDL-C. Likewise, Both CK-MB and cTnI were significantly higher in subjects with AMI than in those with SA/UA (P<0.001). A significant correlation was identified between CK-MB and cTnI level. There was no significant difference between cTnI level and other parameters (Table 2).
Many factors, such as age, gender, smoking, heart failure, hypertension, diabetes, serum creatinine, lipid drugs, and lipid levels, were assessed (Table 3). Among these factors, only the LDL-C level influenced Lp-PLA2 activity. The serum Lp-PLA2activity was higher in the LDL-C>2.6mmol/L group compared with the LDL-C<2.6mmol/L group (P=0.001).
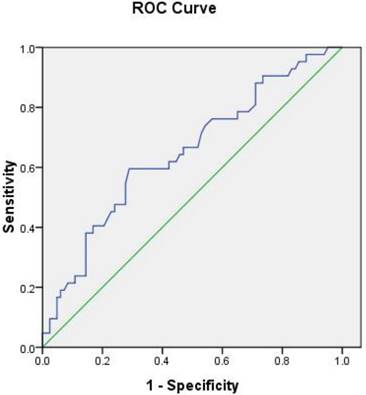
ROC curve analysis for Lp-PLA2 activity
To determine the sensitivity and specificity of Lp-PLA2 levels for diagnosis of AMI, we performed ROC analysis (Fig. 2 and Table 4). The area under the ROC curve (AUC) of Lp-PLA2 activity used to predict AMI was 0.649 (95% CI: 0.546 - 0.752, P=0.007). Based on the ROC curve analysis, the optimal cut-off value for Lp-PLA2 level was 157 IU/L, and the sensitivity and specificity for diagnosis of AMI were 60% and 71%, respectively.
Demographic and laboratory data intotal sample.
| Variable | Total (n=125) | The results of coronary arteriongraphy | P Value | |
|---|---|---|---|---|
| AMI (n=42) | SA/UA (n=83) | |||
| Age, y | 69.7 ±11.2 | 71.1 ±11.1 | 69.0 ±11.3 | 0.413 |
| Gender (male), % | 64.0 (80/45) | 54.8 (23/19) | 68.7 (57/26) | 0.167 |
| Smokers, % | 35.2 (44/81) | 40.5 (17/25) | 32.5 (27/56) | 0.430 |
| Diabetes mellitus, % | 24.0 (30/95) | 21.4 (9/33) | 25.3 (21/62) | 0.825 |
| Hypertension, % | 72.0 (90/35) | 73.8 (31/11) | 71.1 (59/24) | 0.835 |
| NYHA class III or IV, % | 33.3 (62/186) | 61.9 (26/16) | 25.3 (21/62) | 0.000 |
| Lipid drugs medication, % | 83.3 (149/186) | 76.2 (32/10) | 79.5 (66/17) | 0.654 |
| Blood glucose, mmol/L | 5.63 ±1.79 | 5.53 ±1.03 | 5.68 ± 2.08 | 0.285 |
| Tcho, mmol/L | 4.07 ±1.06 | 4.49 ± 1.08 | 3.85 ± 0.99 | 0.003 |
| LDL-C, mmol/L | 2.53 ±0.92 | 2.99 ±0.94 | 2.30 ±0.82 | 0.001 |
| HDL-C, mmol/L | 1.07 ±0.31 | 1.05 ±0.30 | 1.08 ±0.32 | 0.560 |
| Triglycerides, mmol/L | 1.53 ± 0.87 | 1.53 ± 0.72 | 1.52 ±0.95 | 0.649 |
| Serum creatinine, umol/L | 98.1 ±26.9 | 106.3 ± 37.5 | 93.9 ±23.8 | 0.036 |
| CK-MB, IU/L | 28.5±61.7 | 72.8 ± 120.1 | 15.6 ±7.2 | 0.000 |
| cTnI, pg/L | 1.69±1.27 | 7.11 ±16.9 | 0.01 ± 0.01 | 0.000 |
| Lp-PLA2 activity, U/L | 154 ± 54 | 173 ± 59 | 144 ± 46 | 0.007 |
Data are presented as mean ±standard deviation or as number (percentage).
Correlation coefficient of Lp-PLA2 and cTnI with demographic Variables, lipid factors.
| Variable | Lp-PLA2 Correlation coefficient | Pvalue | cTnI Correlation coefficient | Pvalue |
|---|---|---|---|---|
| Lp-PLA2 | - | - | 0.106 | 0.238 |
| cTnI | 0.106 | 0.238 | - | - |
| CK-MB | 0.087 | 0.335 | 0.809 | 0.000 |
| Age | -0.154 | 0.087 | -0,117 | 0.192 |
| Blood glucose | -0.037 | 0.682 | -0.20 | 0.825 |
| Serum creatinine | 0.198 | 0.027 | 0.163 | 0.069 |
| Tcho | 0.361 | 0.000 | 0.037 | 0.684 |
| LDL-C | 0.353 | 0.000 | 0.055 | 0.540 |
| HDL-C | -0.027 | 0.768 | -0.027 | 0.764 |
| Triglycerides | 0.185 | 0.039 | -0.058 | 0.522 |
Factors that affectLp-PLA2 activity intotal sample.
| Categorical Variables | Cases (n=125) | Lp-PLA2 activity, U/L | P Value | |
|---|---|---|---|---|
| Age | ≥65 | 75 | 147±53 | 0.055 |
| <65 | 50 | 162 ±51 | ||
| Gender | male | 80 | 157±54 | 0.494 |
| Female | 45 | 148±50 | ||
| Smokers | Yes | 44 | 164±60 | 0.129 |
| No | 81 | 148±48 | ||
| NYHA grade | I-II | 78 | 149 ± 49 | 0.247 |
| III-IV | 47 | 161 ± 58 | ||
| Diabetes | Yes | 30 | 153±45 | 0.707 |
| No | 95 | 154±55 | ||
| Lipid drugs | Yes | 98 | 153±54 | 0.470 |
| No | 27 | 156±47 | ||
| Creatinine | <97 umol/L | 71 | 149±43 | 0.446 |
| >97 umol/L | 54 | 160±63 | ||
| Tcho | <5.2 mmol/L | 104 | 148±46 | 0.070 |
| >5.2 mmol/L | 21 | 180±71 | ||
| LDL-C | <2.6 mmol/L | 73 | 139±39 | 0.001 |
| >2.6 mmol/L | 52 | 174±63 | ||
| HDL-C | >1 mmol/L | 65 | 150±49 | 0.359 |
| <1 mmol/L | 60 | 158±57 | ||
| Triglycerides | <1.7 mmol/L | 86 | 148±53 | 0.071 |
| >1.7 mmol/L | 39 | 166±51 | ||
Receiver operating characteristic (ROC) curve for various cutoff levels of Lp-PLA2activity in the differentiation of patients withAMI from ACS.
| Lp-PLA2 activity (U/L) | Sensitivity(95%CI) | Specificity(95%CI) | DOC |
|---|---|---|---|
| 134 | 0.71 (0.66-0.76) | 0.47 (0.42-0.52) | 0.60 |
| 145 | 0.62 (0.45-0.54) | 0.58 (0.65-0.74) | 0.57 |
| 157 | 0.60 (0.48-0.56) | 0.71 (0.61-0.70) | 0.51(cutoff) |
| 159 | 0.55 (0.50-0.59) | 0.72 (0.56-0.65) | 0.53 |
| 179 | 0.40 (0.57-0.66) | 0.83 (0.48-0.57) | 0.62 |
*AUC: 0.649 (95% CI: 0.546 - 0.752), P=0.007;
Lp-PLA2,Lipoprotein-Associated Phospholipase A2; DOC, Distance oncurve equaling square root of (1-Sen)2+ (1-Spe) 2; AUC, Area undercurve.
Predictive value of Lp-PLA2 activity for AMI
We divided the study population into two groups according to the cut-off value of Lp-PLA2 activity levels and analyzed the CAD risk factors (Table 5). There were increased incidences of AMI (59% vs 41%, P= 0.001) and higher LDL-C levels (63% vs 37%, P<0.001) in the Lp-PLA2 activity increased group. However, there was no significant difference in the other factors between the two groups.
Risk factors predicted by the cut-off value of Lp-PLA2 activity in the study population.
| Categorical Variables | Lp-PLA2 activity (U/L) | ||
|---|---|---|---|
| ≥157 (n=100) | <157 (n=86) | P value | |
| Age | |||
| <65 | 22 (45%) | 28 (37%) | 0.369 |
| ≥65 | 27 (55%) | 48 (63%) | |
| Gender | 0.807 | ||
| male | 32 (65%) | 48 (63%) | |
| female | 17 (35%) | 28 (37%) | |
| Smokers | 0.502 | ||
| Yes | 19 (39%) | 25 (33%) | |
| No | 30 (61%) | 51 (67%) | |
| Hypertenion | 0.684 | ||
| Yes | 34 (69%) | 56 (74%) | |
| No | 15 (31%) | 20 (26%) | |
| Diabetes | 0.918 | ||
| Yes | 12 (25%) | 18 (24%) | |
| No | 37 (75%) | 58 (76%) | |
| AMI | 0.001 | ||
| Yes | 25 (59%) | 17 (22%) | |
| No | 24 (41%) | 59 (78%) | |
| NYHA grade | 0.330 | ||
| I-II | 28 (57%) | 50 (66%) | |
| III-IV | 21 (43%) | 26 (34%) | |
| LDL-C | 0.000 | ||
| <2.6 mmol/L | 18 (37%) | 55 (72%) | |
| >2.6 mmol/L | 31 (63%) | 21 (28%) | |
| Creatinine | 0.156 | ||
| <97 umol/L | 24 (49%) | 47 (62%) | |
| >97 umol/L | 25 (51%) | 29 (38%) | |
Univariate and Multivariate Logistic Regression Model for Prediction of AMI.
| Variable | Univariate analysis OR (95% CI) | Pvalue | Multivariate analysis OR (95% CI) | Pvalue |
|---|---|---|---|---|
| Lp-PLA2 | 3.615 (1.661-7.868) | 0.001 | 2.723 (1.145-6.473) | 0.023 |
| LDL-C | 3.562 (1.639-7.744) | 0.001 | 2.484 (1.057-5.834) | 0.037 |
| Age | 1.312 (0.610-2.826) | 0.487 | 1.034 (0.400-2.676) | 0.944 |
| Gender | 1.811 (0.843-3.849) | 0.128 | 2.357 (0.840-6.613) | 0.103 |
| Hypertenion | 0.872 (0.378-2.012) | 0.749 | 0.892 (0.356-2.237) | 0.808 |
| Diabetes | 0.805(0.331-1.956) | 0.623 | 0.642 (0.238-1.733) | 0.381 |
| Creatinine | 0.276 (0.717-3.200) | 0.567 | 1.957 (0.764-5.009) | 0.162 |
Using univariate and multivariate logistic regression analysis showed that Lp-PLA2 activity and LDL-C level were significantly associated with AMI (Table 6). In multivariate analysis, Lp-PLA2 activity was associated with the prevalence of AMI with an odds ratio (OR) of 2.723 [95% confidence interval (CI): 1.145‒6.473, p=0.023], and this association was statistically significant (Table 6).
Discussion
Atherosclerotic cardiovascular disease (ASCVD) has a high morbidity and mortality rate increased year by year in China (Oberai et al. 2006). Creatinekinase-MB (CK-MB) and Cardiac troponins (cTn) are the preferred biomarkers to detect myocardial injury, making them promising risk-stratifying tools for patients with symptoms of acute chest pain. They are widely used in clinic because of their high sensitivity, contributed to early diagnosis and treatment of myocardial infarction (McLaurin et al. 1998). Consistent with this, CK-MB and cTnI had a significant correlation, and were both significantly higher in patients with AMI than in those with SA/UA (P<0.001) in our study. However, CK-MB and cTnI are also elevated in other conditions, such as myocarditis, heart failure, fever, renal dysfunction and so on. To compensate for the low specificity, it is required to establish new soluble cardiovascular biomarkers in the bloodstream for AMI prediction.
Major, classic or traditional risk factors of ASCVD are those defined from the Framingham studies, which identified the role of age; sex; total cholesterol, LDL-C and HDL-C levels; systolic pressure; smoking; high blood glucose; body weight; certain dietary habits; and physical inactivity (Bargnoux et al. 2016). A biomarker should be accurate to identify individuals at risk, have reproducible and stable results and, when used early, should have a preventive/therapeutic impact. More recently, new risk biomarkers have been proven to predict the occurrence of cardiovascular events, part of them inflammatory biomarkers that are also involved in the physiopathology of atherosclerotic disease (Chan and Ng 2010).
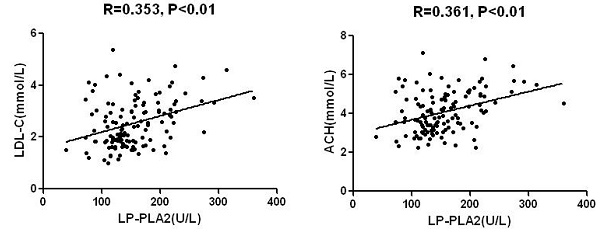
Association between Lp-PLA2 activity and clinical characteristics and lipid parameters.
Inflammation plays a major role in the development and progression of atherosclerosis. ASCVD has an inflammatory character, involving multiple determining factors, including excess body fat, abnormal blood pressure, and glycolipid metabolism. Atherosclerosis begins with endothelial dysfunction, which may be activated by increasing oxidized LDL-C and pro-inflammatory Cytokines (Bonetti et al. 2003). Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a pro-inflammatory enzyme secreted by monocytes and macrophagocytes. Precious study has found that about 70% of Lp-PLA2 binds to low-density lipoprotein (LDL) particles and the residual 30% binds to high-density lipoprotein (HDL) particles (Ge et al. 2016). Our study showed the positive correlation between Lp-PLA2 and LDL-C, not between Lp-PLA2 and HDL-C.
Lp-PLA2 can hydrolyse oxidized phospholipids to yield pro-inflammatory products that are implicated in endothelial dysfunction, plaque inflammation, necrotic core formation inplaque, and rendering plaque vulnerable to rupture. Previous studies found that greater Lp-PLA2 level was independently associated with cardiovascular events in patients with CVD, particularly in patients with stable CVD who were not receiving therapies for inhibiting Lp-PLA2 (Yang et al. 2017). European Guidelines on Cardiovascular Disease (CVD) Preventionin Clinical Practice have made the statement that Lp-PLA2 is an independent vascular specific inflammatory marker and has high consistency and precision as an independent risk factor for plaque rupture and atherothrombotic events (Reiner et al. 2011). There was an inconsistence in the role of Lp-PLA2 for predicting cardiovascular events. Stankovic S and coworkers suggested that the plasma Lp-PLA2 level is an independent predictor of 30-day MACE in patients with first anterior STEMI treated by primary PCI. But Benderly M's results do not support added value of Lp-PLA2 for predicting cardiovascular events or mortality among CHD patients beyond traditional risk factor (Benderly et al. 2017).
Our study demonstrated the correlation between elevated Lp-PLA2 levels and increased risk for both AMI and UA/SA patients. Our results showed that the plasma Lp-PLA2 activity in AMI patients was significantly elevated as compared to that of UA/SA patients, so did the LDL-C level. Although Lp-PLA2 activity was associated with AMI in our data, whether it has clinical utility, it should be added to risk prediction models. We performed ROC analysis to determine the sensitivity and specificity of Lp-PLA2 levels for diagnosis of AMI. Based on the ROC curve analysis, the optimal cut-off value for Lp-PLA2 level was 157 IU/L, and the sensitivity and specificity for diagnosis of AMI were 60% and 71%, respectively, which relatively has high specificity and low sensitivity compared with cTnI that have been used in clinic. Grouped all participants according to Lp-PLA2 cut-off value, there was obviously higher incidences of AMI in the Lp-PLA2 activity over 157 IU/L group. These data suggest Lp-PLA2 is an independent risk factor for AMI and maybe useful complementary to cTnI as a risk predictor of AMI.
ROC Curve Analysis ofLp-PLA2 activity.
Many other factors, which maybe affect Lp-PLA2 activity, such as age, gender, smoking, alcohol consumption, hypertension, diabetes, serum creatinine, lipid drugs, and lipid levels, were assessed. Our study found, age, alcohol consumption, hypertension, and lipid drugs did not affect serum Lp-PLA2 activity. But both Lp-PLA2 activity and LDL-C level were significantly associated with AMI in univariate and multivariate logistic regression analysis. Lp-PLA2 is dependent of LDL-C, but not cTnI, age, gender, BP, DM, which may due to Lp-PLA2 binding to low-density lipoprotein (LDL) particles. Although creatinine level was significantly higher in the AMI patients compared with the UA/SA patients, no correlation was identified between Lp-PLA2 and creatinine. However, cTnI was positively associated with the creatinine level. Lp-PLA2 activity was not affected by renal function and could be a risk predictor of AMI independent to cTnI in renal inadequacy.
Our study has some limitations: (1) it was done in a small, nonrandom sample of subjects; (2) it is a cross-sectional study, and hence causality inferences cannot be made; (3) As numerous comparisons were performed with no adjustment for multiple testing, the increased risk of type I error should be acknowledged.
To summarize, Lp-PLA2 activity positively correlated with LDL-C cholesterol levels in ASCAD and could be an independent risk predictor for AMI. Lp-PLA2 activity was independent of cTnI and may be complementary to cTnI in patients with renal dysfunction.
Acknowledgements
This work was supported by the National Natural Science Foundation (81770505), Research Project of Shanghai Municipal Health and Family Planning Commission (201740060).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38(2):453-459
2. Donato LJ, Meeusen JW, Callanan H, Saenger AK, Jaffe AS. Advantages of the lipoprotein-associated phospholipase A2 activity assay. Clin Biochem. 2015;49(1-2):172-175
3. Acevedo M, Varleta P, Kramer V, Valentino G, Quiroga T, Prieto C, Parada J, Adasme M, Briones L, Navarrete C. Comparison of Lipoprotein-Associated Phospholipase A2 and High Sensitive C-Reactive Protein as Determinants of Metabolic Syndrome in Subjects without Coronary Heart Disease: In Search of the Best Predictor. Int J Endocrinol. 2015;2015:934681
4. Zheng GH, Chen HY, Xiong SQ, Chu JF. Lipoprotein-associated phospholipase A2 gene V279F polymorphisms and coronary heart disease: a meta-analysis. Mol Biol Rep. 2011;38(6):4089-4099
5. Cardinaels EP, Altintas S, Versteylen MO, Joosen IA, Jellema LJ, Wildberger JE, Das M, Crijns HJ, Bekers O, van Dieijen-Visser MP. et al. High-Sensitivity Cardiac Troponin Concentrations in Patients with Chest Discomfort: Is It the Heart or the Kidneys As Well? PloS one. 2016;11(4e):0153300
6. Chung H, Kwon HM, Kim JY, Yoon YW, Rhee J, Choi EY, Min PK, Hong BK, Rim SJ, Yoon JH. et al. Lipoprotein-associated phospholipase A(2) is related to plaque stability and is a potential biomarker for acute coronary syndrome. Yonsei Med J. 2014;55(6):1507-1515
7. Wei L, Ke Z, Zhao Y, Cai Z. The elevated lipoprotein-associated phospholipase A2 activity is associated with the occurrence and recurrence of acute cerebral infarction. Neuroreport. 2017;28(6):325-330
8. Maiolino G, Lenzini L, Pedon L, Cesari M, Seccia TM, Frigo AC, Rossitto G, Caroccia B, Rossi GP. Lipoprotein-associated phospholipase A2 single-nucleotide polymorphisms and cardiovascular events in patients with coronary artery disease. J Cardiovasc Med (Hagerstown). 2015;16(1):29-36
9. Wu X, Zhang Y, Wu Z, You W, Liang F, Ye F, Chen S. Plasma Lipoprotein-Associated Phospholipase A2 Level Is an Independent Predictor of High Thrombus Burden in Patients With Acute ST-segment Elevation Myocardial Infarction. Int Heart J. 2016;57(6):689-696
10. Caslake MJ, Packard CJ, Robertson M, Cooney J, Nelson JJ, Ford I, Gaw A, Jukema JW, Macfarlane PW, Stott DJ. et al. Lipoprotein-associated phospholipase A(2), inflammatory biomarkers, and risk of cardiovascular disease in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). Atherosclerosis. 2010;210(1):28-34
11. Parveen K. Garg, Robyn L. McClelland, Nancy S. Jenny, Michael H. Criqui, Philip Greenland, Robert S. Rosenson, David S. Siscovick, Neal Jorgensen, Mary Cushman. Lipoprotein-associated phospholipase A2 and risk of incident cardiovascular disease in a multi-ethnic cohort: The multi ethnic study of atherosclerosis. Atherosclerosis. 2015;24(1):176-182
12. Garg S, Madhu SV, Suneja S. Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL & glycaemic status in early stages of type-2 diabetes mellitus. Indian J Med Res. 2015;141(1):107-114
13. Oberai PC, Dalal D, Zhang L, Wang C, Eustace J, Parekh RS. Incidence of atherosclerotic cardiovascular disease among HIV patients receiving dialysis. Am J Kidney Dis. 2006;47(5):848-855
14. McLaurin MD, Apple FS, Falahati A, Murakami MM, Miller EA, Sharkey SW. Cardiac troponin I and creatine kinase-MB mass to rule out myocardial injury in hospitalized patients with renal insufficiency. Am J Cardiol. 1998;82(8):973-975
15. Bargnoux AS, Kuster N, Patrier L, Dupuy AM, Tachon G, Maurice F, Badaoui B, Chalabi L, Badiou S, Deleuze S. et al. Cardiovascular risk stratification in hemodialysis patients in the era of highly sensitive troponins: should we choose between hs-troponin I and hs-troponin T? Clin Chem Lab Med. 2016;54(4):673-682
16. Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC medicine. 2010;8:34
17. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168-175
18. Ge PC, Chen ZH, Pan RY, Ding XQ, Liu JY, Jia QW, Liu Z, He SZ, An FH, Li LH. et al. Synergistic Effect of Lipoprotein-Associated Phospholipase A2 with Classical Risk Factors on Coronary Heart Disease: A Multi-Ethnic Study in China. Cell Physiol Biochem. 2016;40(5):953-968
19. Yang L, Liu Y, Wang S, Liu T, Cong H. Association between Lp-PLA2 and coronary heart disease in Chinese patients. J Int Med Res. 2017;45(1):159-169
20. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P. et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-1818
21. Benderly M, Sapir B, Kalter-Leibovici O, Zimlichman R. Lipoprotein-associated phospholipase A2, and subsequent cardiovascular events and mortality among patients with coronary heart disease. Biomarkers. 2017;22(3-4):219-224
Author contact
![]() Corresponding authors: Ting Sun, MD, Department of Cardiology, Shanghai Ninth People's Hospital Affiliated with Shanghai JiaoTong University School of Medicine, Shanghai 200025, China. E-mail: beibeisun2008com and Changqian Wang, MD, Department of Cardiology, Shanghai Ninth People's Hospital Affiliated with Shanghai JiaoTong University School of Medicine, Shanghai 200025, China. E-mail: changqianwangcom
Corresponding authors: Ting Sun, MD, Department of Cardiology, Shanghai Ninth People's Hospital Affiliated with Shanghai JiaoTong University School of Medicine, Shanghai 200025, China. E-mail: beibeisun2008com and Changqian Wang, MD, Department of Cardiology, Shanghai Ninth People's Hospital Affiliated with Shanghai JiaoTong University School of Medicine, Shanghai 200025, China. E-mail: changqianwangcom