J Biomed 2017; 2:101-108. doi:10.7150/jbm.19770 This volume Cite
Research Paper
Anti-inflammatory Effect of JBP485 on Dextran Sulfate Sodium-induced Colitis in Mice
1. Department of Medical Pharmaceutics, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan;
2. Educational Center for Clinical Pharmacy, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan;
3. Department of Organic and Medicinal Chemistry, School of Pharmaceutical Sciences, Aichi Gakuin University, 1-100 Kusumoto-cho, Chikusa-ku, Nagoya 464-8650, Japan;
4. Endoscopy Department, Kindai University Nara Hospital, 1248-1, Otoda-cho, Ikoma 630-0293, Japan;
5. Department of Medical Biochemistry, Kobe Pharmaceutical University, 4-19-1 Motoyamakita-machi, Higashinada-ku, Kobe 658-8558, Japan.
Abstract
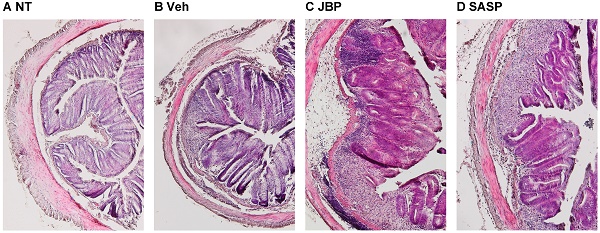
JBP485 (cyclo-trans-4-L-hydroxyprolyl-L-serine), a dipeptide first isolated from Laennec that is a proprietary product made from the hydrolysate of human placenta, has been reported to have several biological properties, such as anti-hepatotoxic, anti-inflammatory and anti-apoptotic activities. However, the effect of JBP485 on inflammatory bowel disease has not yet been reported. Here we investigated the effects of simultaneous and subsequent administration of JBP485 on dextran sulfate sodium (DSS)-induced colitis in mice. In both experimental protocols, DSS-induced colitis was established by giving mice drinking water containing 2% (w/v) DSS for 5 days. JBP485 (25 mg/kg) was orally administered once a day during and after DSS treatment for 5 days in the simultaneous administration experiment while after DSS treatment for 5 days in the subsequent administration experiment. In the simultaneous administration experiment, JBP485 did not improve disease progression, such as weight loss, diarrhea and visible fecal blood, as well as shortening of the colon, but reduced the DSS-induced injury and up-regulation of tumor necrosis factor-α, interleukin-1β, cyclooxygenase-2, monocyte chemotactic protein 1 and C-X-C motif chemokine ligand 1 mRNAs in the colon. In the subsequent administration experiment, however, JBP485 did not show any effects on DSS-induced colitis. These results demonstrated that simultaneous administration but not subsequent administration of JBP485 had anti-inflammatory effects on DSS-induced colitis in mice.
Keywords: dextran sulfate sodium, gene expression, inflammation, inflammatory bowel disease.